Asymmetric catalytic preparation method of brivaracetam
A catalyst and compound technology, applied in the field of asymmetric catalysis preparation of brivaracetam, can solve the problems of high preparation difficulty, increased cost, inability to realize and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
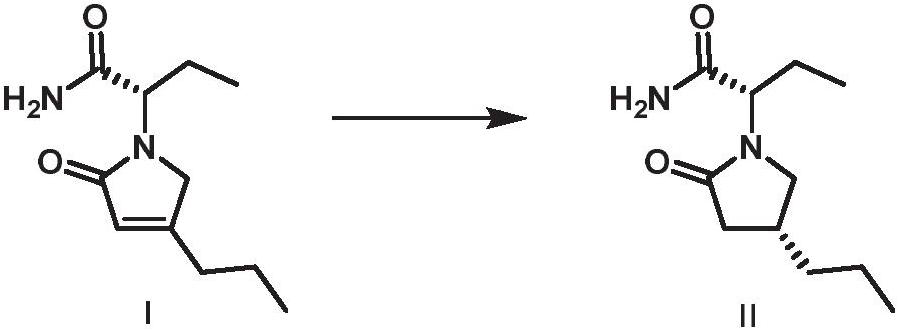
[0025] The preparation method of buvaracetam provided by the invention comprises: the compound of formula (I) undergoes an asymmetric catalytic hydrogenation reaction in a hydrogen atmosphere and an organic solvent in the presence of a rhodium (I) catalyst to generate a compound shown in formula (II) ,
[0026]
[0027] Wherein, the rhodium (I) catalyst is generated after mixing a rhodium metal precursor and a ligand;
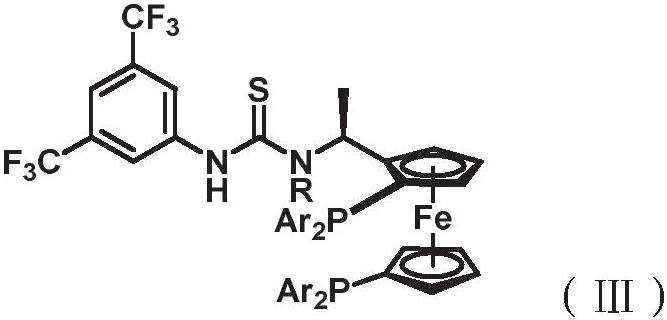
[0028] The ligand has a structure shown in the following formula (III),
[0029]
[0030] in,
[0031] R is H, D, CF 3 or C 1 -C 3 alkyl;
[0032] Ar is aryl or heteroaryl, wherein, each aryl and heteroaryl are optionally selected from one or more independently selected from D, F, Cl, Br, I, CN, NO 2 、CF 3 、C 1 -C 4 Alkyl, C 1 -C 4 Alkoxy groups are substituted.
[0033] In some embodiments, R is H, D, CF 3 , methyl, ethyl or isopropyl;
[0034] In some embodiments, Ar is aryl or heteroaryl, wherein each aryl and heteroaryl are optionally se...
specific Embodiment approach
[0068] In order to make the present invention easy to understand, the present invention will be further described in conjunction with specific embodiments.
[0069] For the experimental methods that do not indicate the specific conditions in the examples, usually follow the conventional conditions and the conditions described in the manual, or according to the conditions suggested by the manufacturer; the materials, reagents, etc. used can be obtained from commercial sources unless otherwise specified. .
Embodiment 1
[0071]
[0072] Into the reaction vessel was added n-heptane (394 mL) and morpholine (128 mL). The mixture was cooled to 0 °C, and glyoxylic acid 1 (195 g, 150 mL, 50 w%, dissolved in water) was added. The mixture was heated at 20°C for 1 hour, then n-valeraldehyde 2 (149 mL) was added. The reaction mixture was heated at 45°C for 20 hours, and after cooling to 20°C, 37% aqueous hydrochloric acid (197 mL) was slowly added to the reaction mixture, and stirring was continued for 2 hours. After the reaction was finished, the n-heptane was removed by liquid separation, and the aqueous phase was washed three times with n-heptane. Diisopropyl ether was added to extract the organic phase, and the organic phases were combined, washed with saturated brine, and dried by azeotropic distillation. After filtering and concentrating the solvent, 165 g of 5-hydroxy-4-n-propylfuran-2-one was obtained, that is, compound 3, and the reaction yield was 88%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com