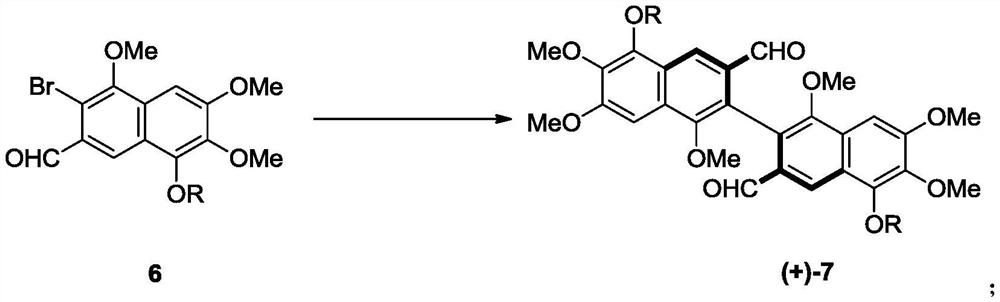
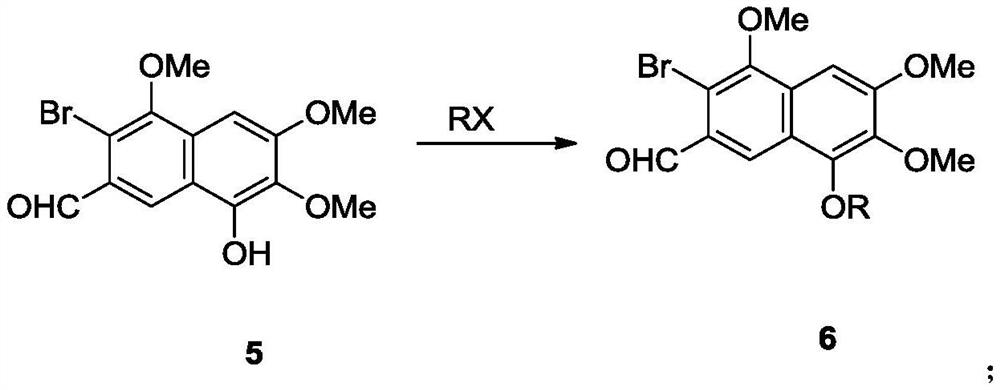
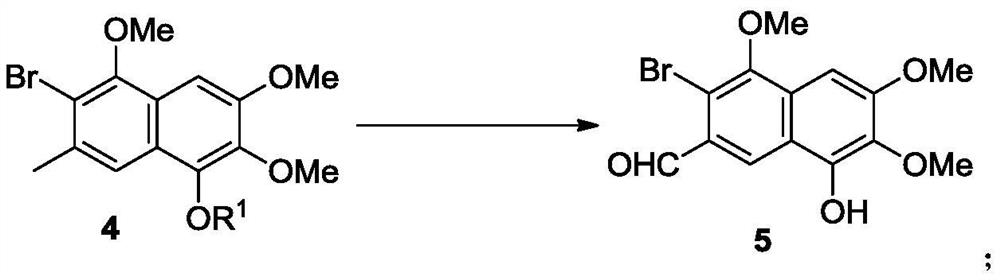
Preparation method and intermediate of gossypol and derivative thereof
A volume and compound technology, applied in the preparation of organic compounds, carbon-based compounds, sulfonate esters, etc., can solve the problems of high cost and cumbersome preparation steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0166] Naphthoquinone compound 3 was prepared by cycloaddition reaction of 1,4-conjugated diene 1 and benzoquinone compound 2.
[0167]
[0168] Among them, the preparation methods of 1,4-conjugated diene 1 and benzoquinone compound 2 refer to Eur.J.Org.Chem., 2000, 1313-1317 and Angew.Chem.Int.Ed., 2015,54, 3792.
[0169] The reaction is as follows: Add 1,4-conjugated diene 1 (13.3 g, 0.05 mol) slowly to a solution of benzoquinone compound 2 (16.8 g, 0.10 mol) in fresh dichloromethane (30 mL) at room temperature The dichloromethane solution (15 mL) was freshly distilled. The addition was complete in about 15 minutes and the resulting solution was stirred at room temperature for 20 hours. Glacial acetic acid (6.85 mL, 0.12 mol) was added to the reaction system, and the resulting solution was stirred at room temperature for 0.5 hours. The crude product obtained after the solution was concentrated under reduced pressure was purified by silica gel column chromatography (pet...
Embodiment 2
[0171] The naphthoquinone compound 3 is reduced to prepare the naphthalene compound 4a in the presence of a reducing agent.
[0172]
[0173] The reaction is as follows: at room temperature, slowly add boron trifluoride diethyl ether solution (27 mL, 0.10 mol) and triethylsilane (15.9 mL, 0.1 mol). The addition was complete in about 30 minutes and the resulting solution was stirred at room temperature for 15 hours. At 0°C, acetyl chloride (7.1 mL, 0.10 mol) was added to the reaction system, and the resulting solution was warmed to room temperature in 0.5 hours and stirred for 6 hours. Add deionized water (50mL) to the reaction solution, stir well and separate the organic phase, extract the aqueous phase with dichloromethane (20mL×3), combine the organic phases, dry over anhydrous sodium sulfate, and spin dry with a rotary evaporator After purification by silica gel column chromatography (petroleum ether: ethyl acetate = 8:1), a light yellow solid 4a (13.2 g, yield 72%) wa...
Embodiment 3
[0175] Compound 4a was converted to naphthol compound 5 in the presence of brominating reagents and oxidizing agents.
[0176]
[0177] The reaction was as follows: at 80°C, the free radical initiator AIBN (0.82 g, 0.005mol). The resulting solution was stirred at 80°C for 8 hours. N-methylmorpholine-N-oxide (23.4 g, 0.2 mol) was added to the reaction system. The resulting solution was stirred for a further 12 hours at 80°C. Add 3N hydrochloric acid solution (100mL) and dichloromethane (100mL) to the reaction solution, stir well and separate the organic phase, extract the aqueous phase with dichloromethane (30mL×3), combine the organic phases, and dry over anhydrous sodium sulfate , and spin-dried with a rotary evaporator to obtain light yellow solid 5 (13.2 g, yield 72%). 1 H NMR (600MHz, CDCl 3 )δ10.43(s,1H),8.54(s,1H),7.03(s,1H),6.41(s,1H),4.05(s,3H),4.01(s,3H),3.99(s,3H ); 13 C NMR (151MHz, CDCl 3 ) δ 191.8, 155.6, 152.3, 146.5, 134.3, 129.4, 128.6, 122.7, 119.5,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com