Preparation and application of 5-aminolevulinic acid-camptothecin micromolecular prodrug
A technology of aminolevulinic acid and camptothecin, which is applied in the directions of non-active ingredients medical preparations, active ingredients-containing medical preparations, pharmaceutical formulas, etc., can solve the problem of poor selectivity, low efficacy of single chemotherapy, and low drug load. And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1 Preparation of a 5-aminolevulinic acid-camptothecin small molecule prodrug
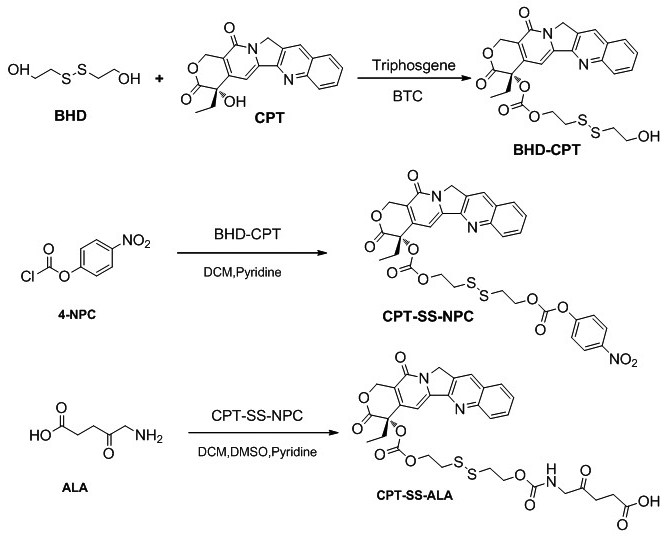
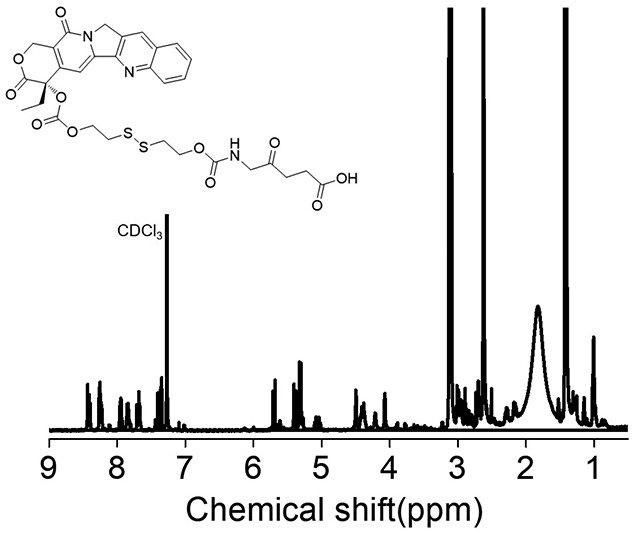
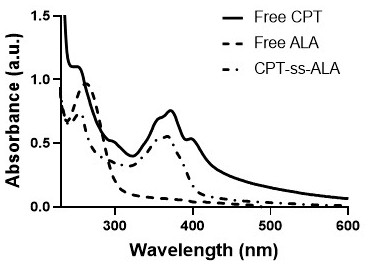
[0028] A kind of total synthetic schematic diagram of 5-aminolevulinic acid-camptothecin small molecule prodrug as figure 1 As shown, it mainly includes the following steps:
[0029] (1) Preparation of camptothecin CPT precursor BHD-CPT with disulfide bonds, the synthesis route is shown below, including the following steps: under the conditions of 25°C and 2-10Pa argon atmosphere, camptothecin CPT ( 376.2mg, 1.08mmol) and 4-lutidine DMAP (420.6mg, 3.42mmol) were dissolved in anhydrous dichloromethane DCM (15mL); triphosgene BTC (123mg, 0.396mmol) was dissolved in anhydrous DCM ( 15mL), then the BTC solution dissolved in anhydrous DCM was added dropwise to the reaction system, and the reaction was protected from light for 0.5-1h; 2-hydroxyethyl disulfide BHD (830mg, 5.4mmol) was dissolved in anhydrous tetrahydrofuran THF (4mL), then slowly drop the BHD solution dissolved in anhydrous...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com