A kind of large-scale synthetic method of ajirelin
A synthetic method, the technology of dimethylformamide, applied in the field of peptide synthesis, can solve the problems of long synthesis period, high carrier cost, and low loading capacity, and achieve the effects of increased yield, reduced difficulty, and increased total yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
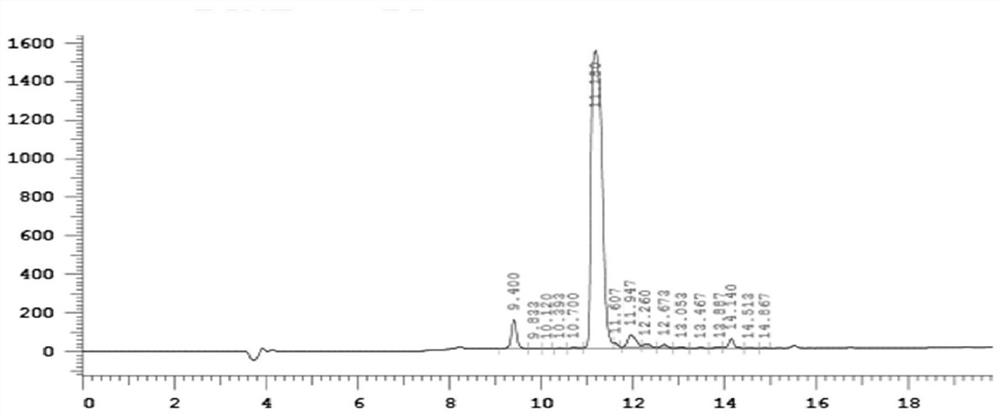
Image
Examples
Embodiment 1
[0048] Embodiment 1: a kind of large-scale synthesis method of ajirelin
[0049] The large-scale synthetic method of ajirelin is as follows:
[0050] (1) Synthesis of Ac-Glu(OtBu)-Glu(OtBu)-Met-Gln(Trt)-Arg(Pbf)-Arg(Pbf)-2-Chlorotrityl Chloride Resin;
[0051] (2) Synthesis of Ac-Glu(OtBu)-Glu(OtBu)-Met-Gln(Trt)-Arg(Pbf)-Arg(Pbf)-OH;
[0052] (3) Synthesis of Ac-Glu(OtBu)-Glu(OtBu)-Met-Gln(Trt)-Arg(Pbf)-Arg(Pbf)-NH 2 ;
[0053] (4) Synthesis of Ac-Glu-Glu-Met-Gln-Arg-Arg-NH 2 .
Embodiment 2
[0054] Embodiment 2: Synthesis of Ac-Glu(OtBu)-Glu(OtBu)-Met-Gln(Trt)-Arg(Pbf)-Arg(Pbf)-2-Chlorotrityl Chloride Resin
[0055] On the basis of the technical solution provided in Example 1, this example further revolves around Ac-Glu(OtBu)-Glu(OtBu)-Met-Gln(Trt)-Arg(Pbf)-Arg(Pbf)-2-Chlorotrityl Chloride Resin The synthesis of the specific synthesis steps are as follows:
[0056] (1) Synthesis of Fmoc-Arg(Pbf)-2-Chlorotrityl Chloride Resin
[0057] Add 100.0g 2-Chlorotrityl Chloride Resin to the synthesizer, add 1000mL dichloromethane to soak and swell for 15 minutes, then filter with suction, add 1000mL dichloromethane, 114.2g (0.176mol) Fmoc-Arg(Pbf) to the synthesizer -OH, 105ml (0.64mol) N,N´-diisopropylethylamine, under the protection of nitrogen, stirred at room temperature for 2 hours, blocked with anhydrous methanol for 0.5 hours, filtered with suction, and washed twice with isopropanol ( Wash twice with 800ml each time) and N,N-dimethylformamide (800ml each time) to o...
Embodiment 3
[0064] Example 3: Synthesis of Ac-Glu(OtBu)-Glu(OtBu)-Met-Gln(Trt)-Arg(Pbf)-Arg(Pbf)-OH
[0065] On the basis of the technical solution provided in Example 1, this example further revolves around the synthesis of Ac-Glu(OtBu)-Glu(OtBu)-Met-Gln(Trt)-Arg(Pbf)-Arg(Pbf)-OH, specifically The synthesis steps are as follows:
[0066] Add the dried Ac-Glu(OtBu)-Glu(OtBu)-Met-Gln(Trt)-Arg(Pbf)-Arg(Pbf)-2-Chlorotrityl Chloride Resin into a 2L beaker, add 200ml of trifluoroethanol, Acetic acid 75ml, dichloromethane 725ml cutting solution, cracking resin, stirring reaction for 2 hours, filtering, filter cake washed three times with dichloromethane, each 100ml, combined filtrate, filtrate was washed twice with saturated sodium bicarbonate solution, each 700ml Use saturated sodium bicarbonate solution to pH = 6, adjust pH = 7 with 800ml of saturated saline, combine the water phase and filtrate into an eggplant-shaped bottle, add 500ml of saturated saline, remove the organic solvent under r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com