Method for synthesizing aromatic trifluoromethylthio compound
A technology for synthesizing aromatic hydrocarbons based on trifluoromethylthio group, which can be used in organic chemistry, thioether preparation and other directions, and can solve the problems of environmental problems of fluorine-containing reagents, high price, and limited substrate scope.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] in N 2 Under protection, add diphenyl phosphorus chloride (2.20g, 10mmol) and sodium trifluoromethanesulfinate (1.56g, 10mmol), add 20mLMeCN, stir at room temperature under airtight condition for 30min, then in N 2 4-Phenylaniline (1.69g, 10mmol), tert-butyl nitrite (1.54g, 15mmol) and anhydrous CuSO were sequentially added under protection. 4 (0.32g, 2mmol), heated to 70°C under airtight conditions, stirred at constant temperature for 12h, cooled to room temperature after fully reacting, the reaction solution was extracted 3 times with a mixture of dichloromethane and water, the organic layer was separated and dried over anhydrous sodium sulfate Then the solvent was distilled off under reduced pressure. The crude product was separated by column chromatography (the eluent was a mixture of ethyl acetate and petroleum ether) to obtain the 4-phenyl-1-trifluoromethylthiobenzene product with a yield of 58%.
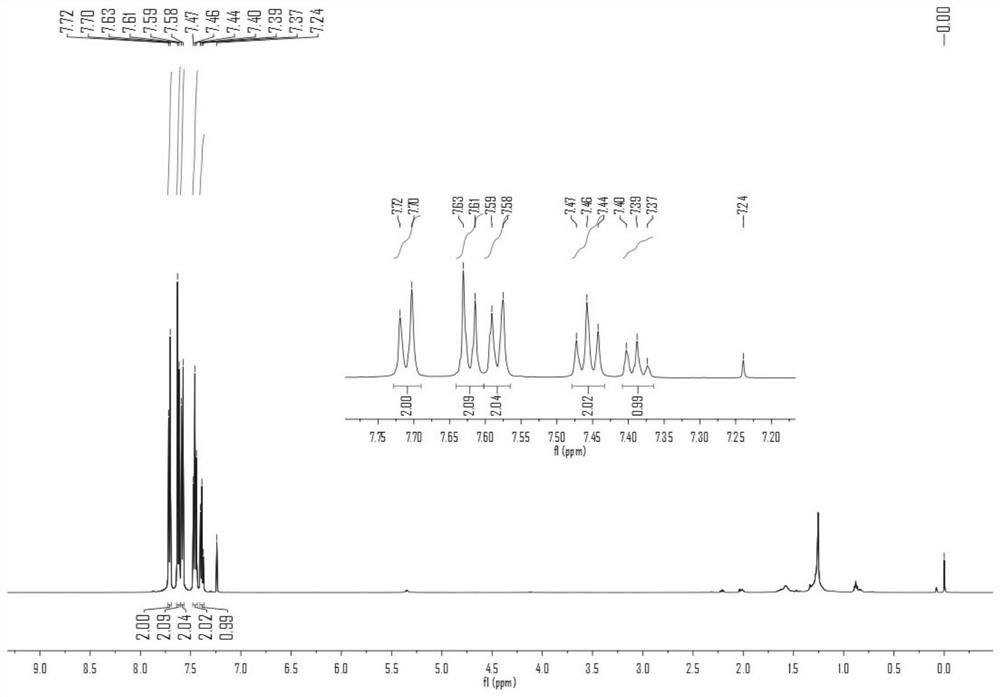
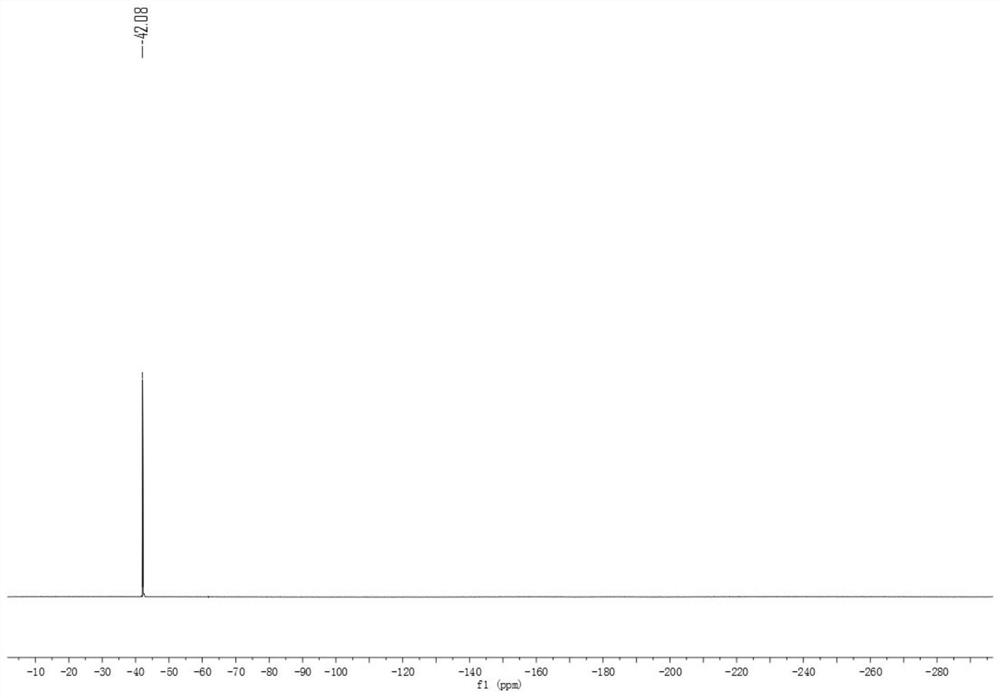
[0039] 4-Phenyl-1-trifluoromethylthiobenzene 1 H NMR see figur...
Embodiment 2
[0043] in N 2 Under protection, add diphenyl phosphorus chloride (2.20g, 10mmol) and sodium trifluoromethanesulfinate (1.56g, 10mmol), add 20mLMeCN, stir at room temperature under airtight condition for 30min, then in N 2 Under protection, 4-cyclohexylaniline (1.69g, 10mmol), tert-butyl nitrite (1.54g, 15mmol) and anhydrous CuSO were added successively. 4(0.32g, 2mmol), heated to 70°C under airtight condition, stirred at constant temperature for 12h, fully reacted and cooled to room temperature, the reaction solution was extracted 3 times with a mixture of dichloromethane and water, the organic layer was separated and dried over anhydrous sodium sulfate Then the solvent was distilled off under reduced pressure. The crude product was separated by column chromatography (the eluent was a mixture of ethyl acetate and petroleum ether) to obtain the 4-cyclohexyl-1-trifluoromethylthiobenzene product with a yield of 65%.
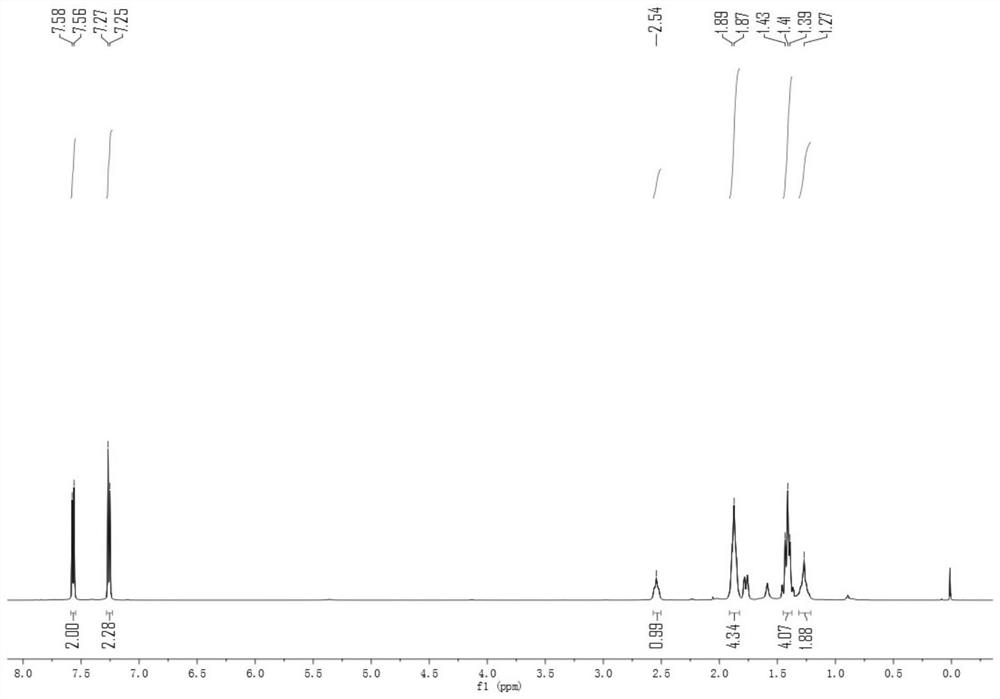
[0044] 4-Cyclohexyl-1-trifluoromethylthiobenzene 1 H NMR se...
Embodiment 3
[0048] in N 2 Under protection, add diphenyl phosphorus chloride (2.20g, 10mmol) and sodium trifluoromethanesulfinate (1.56g, 10mmol), add 20mLMeCN, stir at room temperature under airtight condition for 30min, then in N 2 Under protection, 4-cyanoaniline (1.69g, 10mmol), tert-butyl nitrite (1.54g, 15mmol) and anhydrous CuSO were added successively. 4 (0.32g, 2mmol), heated to 70°C under airtight condition, stirred at constant temperature for 12h, fully reacted and cooled to room temperature, the reaction solution was extracted 3 times with a mixture of dichloromethane and water, the organic layer was separated and dried over anhydrous sodium sulfate Then the solvent was distilled off under reduced pressure. The crude product was separated by column chromatography (the eluent was a mixture of ethyl acetate and petroleum ether) to obtain the 4-cyano-1-trifluoromethylthiobenzene product with a yield of 55%.
[0049] 4-cyano-1-trifluoromethylthio 1 H NMR see Figure 5 , 4-cyan...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com