Carbazole-containing compound and organic electroluminescent device thereof
A compound and carbazole technology, applied in the field of carbazole-containing compounds and organic electroluminescent devices, can solve the problems of poor application effect of blue light devices, adverse effects of organic materials, poor practicability, etc., to avoid adverse effects, improve Excellent luminous efficiency, stability and durability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0071] Preparation of Intermediate C:
[0072]
[0073] Compound A and compound B reacted through Suzuki coupling to obtain intermediate C;
[0074] Preparation of Intermediate E:
[0075]
[0076] Intermediate C and compound D were reacted by Suzuki coupling to obtain intermediate E;
[0077] The preparation of the compound containing carbazole shown in general formula (I):
[0078]
[0079] Intermediate E reacts with compound F via Buchwald-Hartwig to obtain a carbazole-containing compound represented by general formula (I).
[0080] Among them, the X 1 、X 2 independently selected from an oxygen atom or a sulfur atom;
[0081] Ar is selected from alkyl groups with 1 to 12 carbon atoms, aryl groups with 6 to 30 carbon atoms, heteroaryl groups with 3 to 30 carbon atoms, -NR 3 R 4 one of the R 3 , R 4 Independently selected from one of an aryl group with 6 to 30 carbon atoms and a heteroaryl group with 3 to 30 carbon atoms, the heteroaryl group contains at lea...
Synthetic example 1
[0105] Synthesis Example 1: Synthesis of Compound A
[0106] Synthesis of compound A-1:
[0107]
[0108] 3.25g (10mmol) 3,6-dibromocarbazole, 6.10g (24mmol) biboronic acid pinacol ester, 0.50g (0.6mmol) 1,1'-bis(diphenylphosphine)-ferrocene- Palladium dichloride (II) dichloromethane complex and 5.90 g (60 mmol) of potassium acetate were dissolved in 80 ml of toluene, and the reaction was carried out under reflux for 10 hours. Cool to room temperature, wash with distilled water, extract with toluene, filter the organic phase with a diatomaceous earth bed, distill off the organic solvent under reduced pressure, and recrystallize in heptane / toluene to obtain 3.73g (8.9mmol) of compound A-1, HPLC purity 99.5%, yield 89%.
[0109] Synthesis of compound A-2:
[0110]
[0111] Replace 3,6-dibromocarbazole with an equimolar amount of 2,7-dibromocarbazole, and the other steps are the same as those of compound A-1 to obtain 3.65g (8.7mmol) of compound A-2 , the HPLC purity wa...
Synthetic example 2
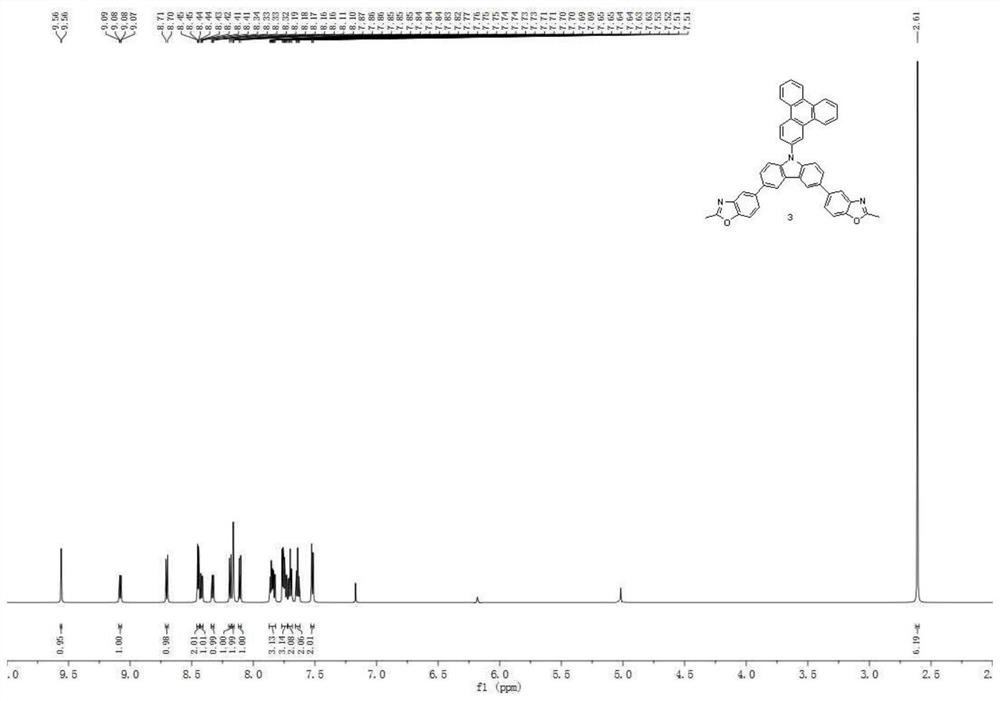
[0112] Synthesis Example 2: Synthesis of Compound 3
[0113]
[0114] 2.10g (5mmol) compound A-1, 2.12g (10mmol) compound B-1, 0.23g (0.2mmol) tetrakis (triphenylphosphine) palladium are dissolved in the mixed solvent of 30ml toluene and 15ml sodium carbonate aqueous solution (2M) In, reflux reaction for 8 hours. Cool to room temperature, extract with toluene, wash the organic phase with saturated brine, dry, and purify by column chromatography to obtain 1.67 g (3.9 mmol) of compound E-1, the HPLC purity is 99.0%, and the yield is 78%.
[0115] 0.86g (2mmol) compound E-1 was dissolved in the NMP of 20ml, then added 0.80g (2.6mmol) compound F-1, 0.28g (2mmol) sodium sulfate, 0.28g (2mmol) potassium carbonate and 0.04g (0.6 mmol) copper, reacted at 200°C for 24 hours. Cool to room temperature, remove the solvent by distillation under reduced pressure, wash with distilled water, and extract with dichloromethane, dry the organic phase with anhydrous magnesium sulfate, then re...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com