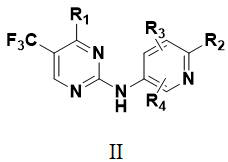
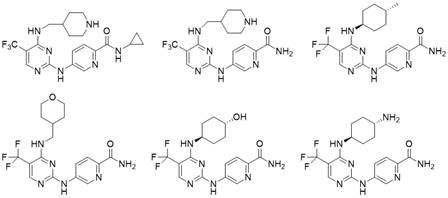
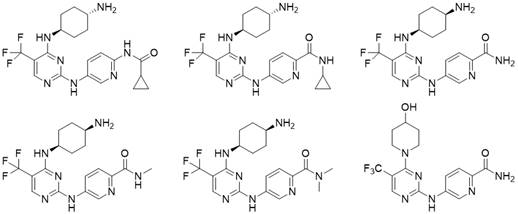
no 2 -Carbamoyl aromatic ring-2-aminopyrimidine derivatives and their medical use
A carbamoyl aromatic ring and aminopyrimidine technology, applied in the application field of antitumor drugs, can solve the problems of off-target, secondary drug resistance, poor monotherapy effect, etc., to reduce toxic side effects and overcome drug resistance. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0145] Preparation Example 1 Preparation of Compounds 1 to 7
[0146]
[0147] Step 1. 4-Chloro-5-trifluoromethyl-2-aminopyrimidine (1-2)
[0148]
[0149] Dissolve 2,4-dichloro-5-trifluoromethylpyrimidine (6.5 g, 30.0 mmol) in acetonitrile (30 mL), slowly add ammonia water (20 mL) dropwise under ice bath, move to room temperature to react for 40 min, reduce The solvent was recovered by pressure distillation to obtain a residue. Purified by silica gel column chromatography with PE:EA (6:1) as eluent to obtain intermediate 1-2 as a white solid. Yield: 46%; 1 H NMR (500MHz, CDCl 3 ) δ8.47(s, Ar-H, 1H), 5.57(s, NH, 2H); ESI-MS: m / z=217[M+H] + .
[0150] Step 2. 1-Boc-4-(((2-amino-5-(trifluoromethyl)pyrimidin-4-yl)amino)methyl)piperidine (1-3)
[0151]
[0152] Intermediate 1-2 (593mg, 3.0mmol) was dissolved in anhydrous methanol (12mL), triethylamine (303mg, 3.0mmol), 1-Boc-4-aminomethylpiperidine (771mg, 3.6mmol) were added successively , heated to reflux for 8h,...
preparation Embodiment 2
[0171] Preparation Example 2 Preparation of Compounds 8-16
[0172]
[0173] Step 1. Synthesis of compounds 1-12~1-19
[0174] Synthesis steps Referring to step 2 of Example 1, compounds 1-12 to 1-19 were prepared by substituting the corresponding amine for 1-Boc-4-aminomethylpiperidine.
[0175] 1.1N 4 -(Tetrahydropyran-4-ylmethyl)-5-trifluoromethylpyrimidine-2,4-diamine (1-12) 1 H NMR (500MHz, CDCl 3 )δ8.05(s,Ar-H,1H),5.35–4.99(m,s,NH×3,3H),4.00–3.97(m,CH 2 ,2H),3.42–3.34(m,CH 2 ×2,4H),1.91–1.82(m,CH,1H),1.65–1.60(m,CH 2 ,2H),1.38–1.30(m,CH 2 ,2H); ESI-MS: m / z=277[M+1] + .
[0176] 1.2 1-Boc-N 4 -(1r,4r)-4-Aminocyclohexyl-5-trifluoromethylpyrimidine-2,4-diamine (1-13) 1 H NMR (500 MHz, DMSO-d 6 )δ7.96(s, Ar-H, 1H), 6.76(d, J=8.5Hz, NH 2 ,NH 2 -a,3H),6.05(d,J=8.5Hz, NH 2 -b, 1H), 4.19–3.94 (m, CH, 1H), 3.22–3.15 (m, CH, 1H), 1.78–1.73 (m, CH 2 ×2,4H),1.55– 1.42(m,CH 2 ,2H),1.37(s,CH 3 ×3,9H),1.24–1.16(m,CH 2 , 2H); ESI-MS: m / z=376[M+1] + .
[0177] 1.3 ...
preparation Embodiment 3
[0194] Preparation Example 3 Preparation of Compounds 17-19
[0195]
[0196] Step 1. Preparation of Intermediates 1-21 to 1-22
[0197] Synthetic steps refer to step 3 of Example 1, and the corresponding amines are used to prepare compounds 1-21 to 1-22.
[0198] 1.1 5-Bromo-N-picolinamide (Intermediate 1-21) 1 H NMR (500MHz, CDCl 3 )δ8.58(d,J=2.0Hz,Ar-H,1H),8.08(d,J=8.5Hz,Ar-H,1H),7.96(dd,J=8.5,2.0Hz,Ar-H, 1H), 7.93–7.84(m, NH, 1H), 3.02(d, J=5.0Hz, CH 3 ,3H).ESI-MS: m / z=215[M+1] + .
[0199] 1.2 5-Bromo-N,N-lutidine amide (Intermediate 1-22) 1 H NMR (500MHz, CDCl 3 )δ8.63(d, J=2.0Hz,Ar-H,1H),7.92(dd,J=8.5,2.0Hz,Ar-H,1H),7.57(dd,J=8.5,2.0Hz,Ar- H,1H),3.12(s,CH 3 ,3H),3.09(s,CH 3 ,3H).ESI-MS: m / z=229[M+1] + .
[0200] Step 2. Preparation of Compounds 17-19
[0201] For the synthesis steps, refer to step 4 of Preparation Example 1, replace 1-3 with 1-13 / 1-14, replace 1-5-1-11 with 1-21-1-22 / 1-9, and prepare compounds 17-19 .
[0202] 2.1N-(5-((4-(((1r,4r)-4-a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com