Preparation method of CuCo-N/C nano-catalyst and application thereof in preparation of lactic acid by catalytic oxidation of 1,2-propylene glycol
A nano-catalyst and catalyst technology, applied in the field of catalysis, can solve the problems of high cost and limited application expansion, and achieve the effect of energy saving and high application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] (1) Prepare Regenerated Silk Fibroin Solution (RSF):
[0027] Cut 10 grams of silkworm cocoons into sheets of uniform size, add them to the prepared 0.02M anhydrous sodium carbonate solution and boil for 30 minutes to remove the sericin and wax in the silkworm cocoons to obtain regenerated silk fibroin. Wash the regenerated silk fibroin with deionized water three times and dry naturally at room temperature. Weigh 2.5g of regenerated silk fibroin and add it to 10ml of 9.3M lithium bromide solution, keep it in an oven at 60℃ for 4 hours, after completely dissolving, dialyzed with deionized water for three days to remove lithium bromide, and centrifuge to remove insoluble impurities to obtain regenerated silk Element solution (RSF); the volume of the solution is 40 ml, and the mass concentration of RSF is 5%.
[0028] (2) Preparation of Cu-Co bimetallic nanoparticle colloid:
[0029] 0.415g Cu(NO 3 ) 2 ·2H 2 O, 0.395g Co(NO 3 ) 2 ·6H 2 Dissolve O in 40mL 5% RSF solution, adjust...
Embodiment 2
[0035] (1) Preparation of Regenerated Silk Fibroin Solution (RSF): Step (1) is the same as in Example 1
[0036] (2) Preparation of Cu-Co bimetallic nanoparticle colloid:
[0037] 0.415g Cu(NO 3 ) 2 ·2H 2 O, 0.395g Co(NO 3 ) 2 ·6H 2 Dissolve O in 40mL 5% RSF solution, adjust the pH of the mixed solution to 11.03, after the solution turns purple-blue, stir and react at room temperature for 24h to obtain Cu-Co bimetallic nanoparticle colloid;
[0038] (3) Preparation of CuCo-N / C doped bimetallic nanocatalyst by freeze-drying and carbonization:
[0039] The prepared Cu-Co bimetallic nanoparticle colloid was freeze-dried at -85°C for 4 days, and then removed at 5°C for min -1 The temperature increase rate of the temperature rise is raised to 800°C in a tube furnace and kept for 4 hours, and after cooling, a 10% CuCo-N / C800 doped bimetallic nano-catalyst is obtained.
[0040] (4) Catalytic oxidation reaction of 1,2-propanediol:
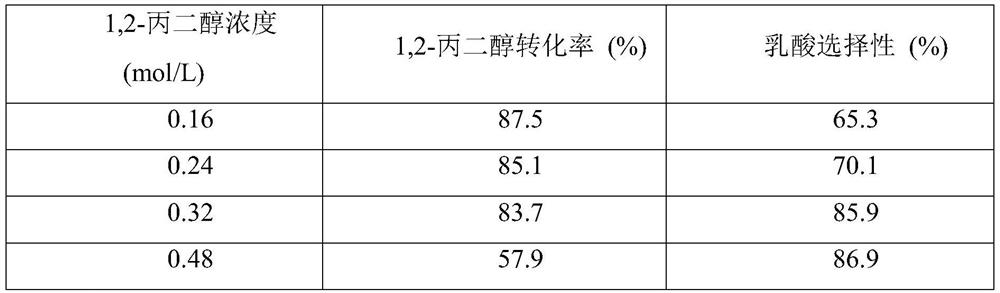
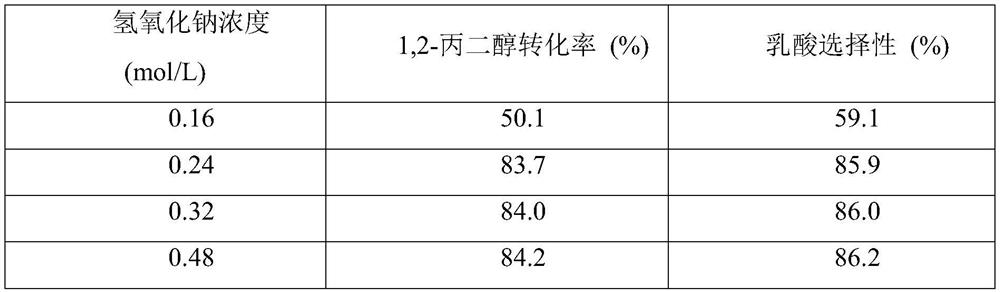
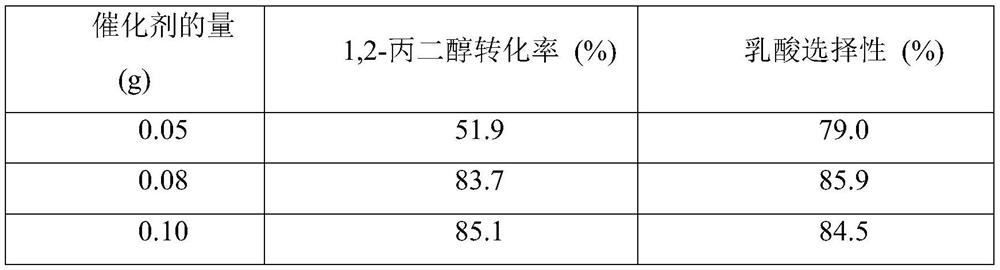
[0041] First, the prepared 40mL 0.32mol / L 1,2-propanediol, 0.24...
Embodiment 3
[0043] (1) Preparation of Regenerated Silk Fibroin Solution (RSF): Step (1) is the same as in Example 1
[0044] (2) Preparation of Cu-Co bimetallic nanoparticle colloid:
[0045] 0.415g Cu(NO 3 ) 2 ·2H 2 O, 0.395g Co(NO 3 ) 2 ·6H 2 Dissolve O in 40mL 5% RSF solution, adjust the pH of the mixed solution to 10.86, after the solution turns purple-blue, stir and react at room temperature for 24h to obtain Cu-Co bimetallic nanoparticle colloid;
[0046] (3) Preparation of CuCo-N / C doped bimetallic nanocatalyst by freeze-drying and carbonization:
[0047] The prepared Cu-Co bimetallic nanoparticle colloid was freeze-dried at -85°C for 5 days, and then removed at 5°C for min -1 The temperature rise rate is raised to 1000° C. in a tube furnace and kept for 4 hours, and after cooling, a 10% CuCo-N / C1000 doped bimetallic nano-catalyst is obtained.
[0048] (4) Catalytic oxidation reaction of 1,2-propanediol:
[0049] First, the prepared 40mL 0.32mol / L 1,2-propanediol, 0.24mol / L sodium hydroxide a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com