An optimized method for programmed cooling of umbilical cord blood hematopoietic stem cells
A technology of hematopoietic stem cells and umbilical cord blood, applied in the biological field, can solve the problems of program-controlled cooling, lack of control of the temperature of umbilical cord blood samples, and large differences in the quality of umbilical cord blood cryopreservation, so as to ensure contact and penetration, maintain proliferation ability, and good proliferation effect of ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] This embodiment provides an optimized method for programmed cooling of umbilical cord blood hematopoietic stem cells, comprising the following steps:
[0041] S1, the cord blood after collection should be prepared and separated within 24 hours to remove red blood cells and plasma, and retain the nucleated cell components rich in hematopoietic stem cells, which is the cord blood sample; at the same time, the cord blood selected and to be frozen has similar The waste umbilical cord blood with nuclear cell count and hematocrit is prepared according to the same method as the umbilical cord blood sample and cryoprotectant is added, as a reference sample for subsequent program-controlled cooling;
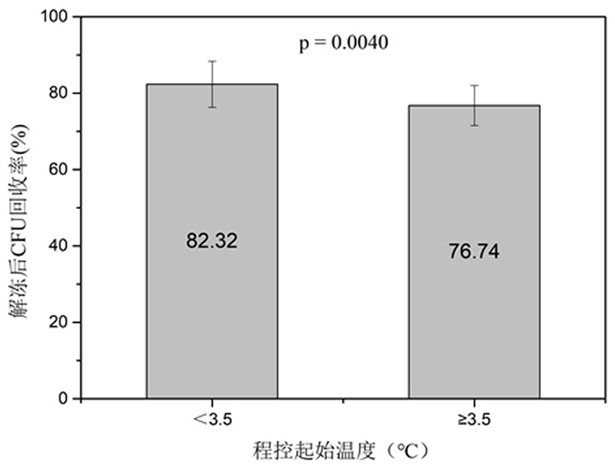
[0042] S2, the cord blood sample and the cryoprotectant were pre-cooled at 4°C for 10 minutes, and then the cryoprotectant was added to the cord blood sample obtained in step 1) using a micro-injection pump, and the cord blood sample was added during the addition process Put it in ...
Embodiment 2
[0054]This embodiment is an improvement of the above-mentioned embodiment 1, and it is a refinement of the above-mentioned embodiment about the cryoprotectant. The cryoprotectant in this embodiment is a mixture of dimethyl sulfoxide and dextran 40, and the cryoprotectant The added volume was 1 / 4 of the volume of the cord blood sample; the cryoprotectant contained 50% (v / v) dimethyl sulfoxide (DMSO) and 5% (w / v) dextran 40.
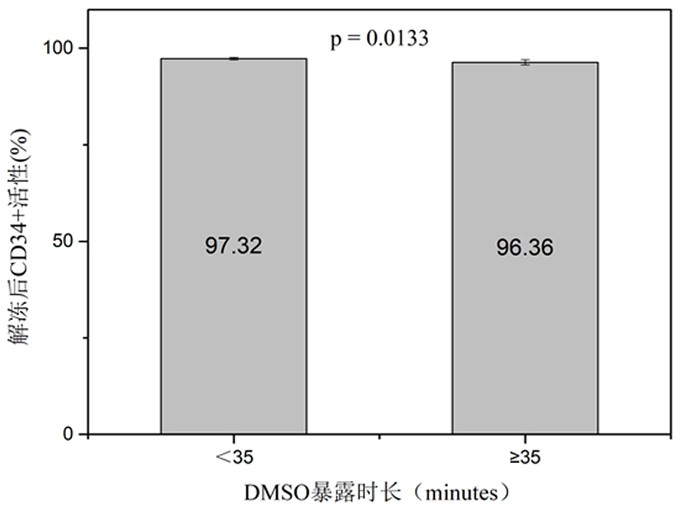
[0055] Since DMSO is toxic to cells at room temperature, both the cord blood specimen and the cryoprotectant are pre-cooled in a 4°C refrigerator for 10 minutes in advance to ensure that both the cryoprotectant and the cord blood specimen are in a low temperature state when in contact, avoiding DMSO damage to cells.
[0056] Strictly control the addition process of the cryoprotectant. One is to use a micro-injection pump to ensure slow and uniform addition, and the control time is 10-15 minutes. The second is to ensure a low temperature environment of 0-4°...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com