Isolated novel coronavirus monoclonal antibody or antigen binding part thereof
A monoclonal antibody, coronavirus technology, applied in the fields of molecular biology and cellular immunology, can solve problems such as complex components
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] Example 1 Antibody Screening
[0084] 1. Phage library construction
[0085] 1. Collect peripheral blood from patients with COVID-19 during recovery period, and separate mononuclear cells (PBMC) from peripheral blood
[0086]In this project, on February 14, 2020, after informed consent, 20ml of peripheral blood was collected from 5 patients diagnosed with COVID-19 before discharge. The 5 patients belonged to the same transmission chain, and none of them were severe cases. After treatment, they were discharged from the hospital from February 15th to 22nd and were isolated at home. Mononuclear cells (PBMCs) in 20 ml of heparin-anticoagulated blood were separated by density gradient centrifugation using GE's Ficoll-Paque PLUS.
[0087] 2. RNA extraction and cDNA synthesis in PBMC
[0088] Use QIAGEN's RNeasy Mini Kit to extract PBMC cell RNA, and then use Roche's first strand synthesis kit (Transcriptor First Strand cDNA Synthesis Kit, Roche, Cat No.: 04896866001) to re...
Embodiment 2
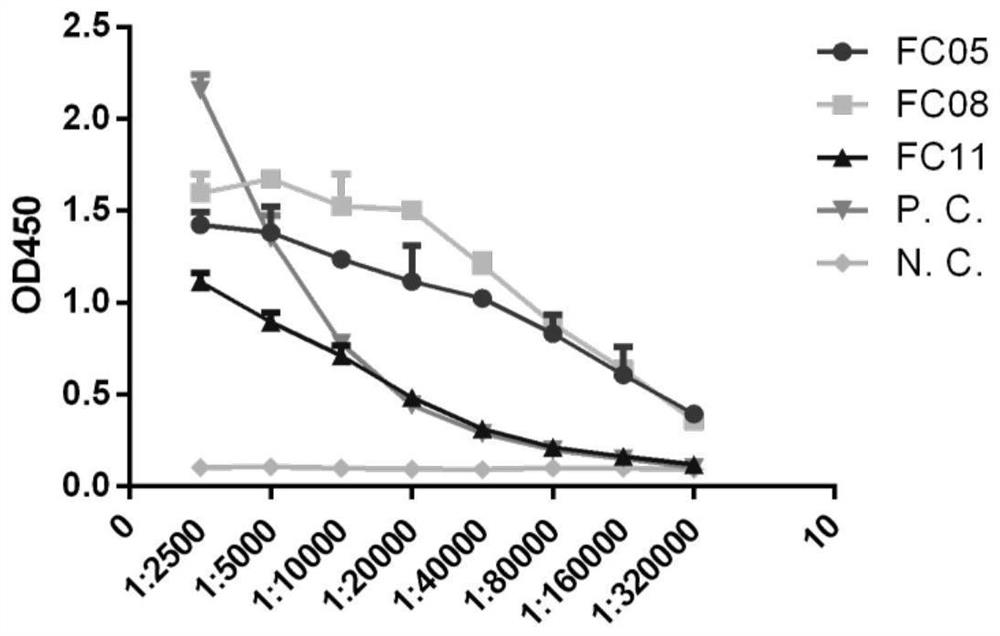
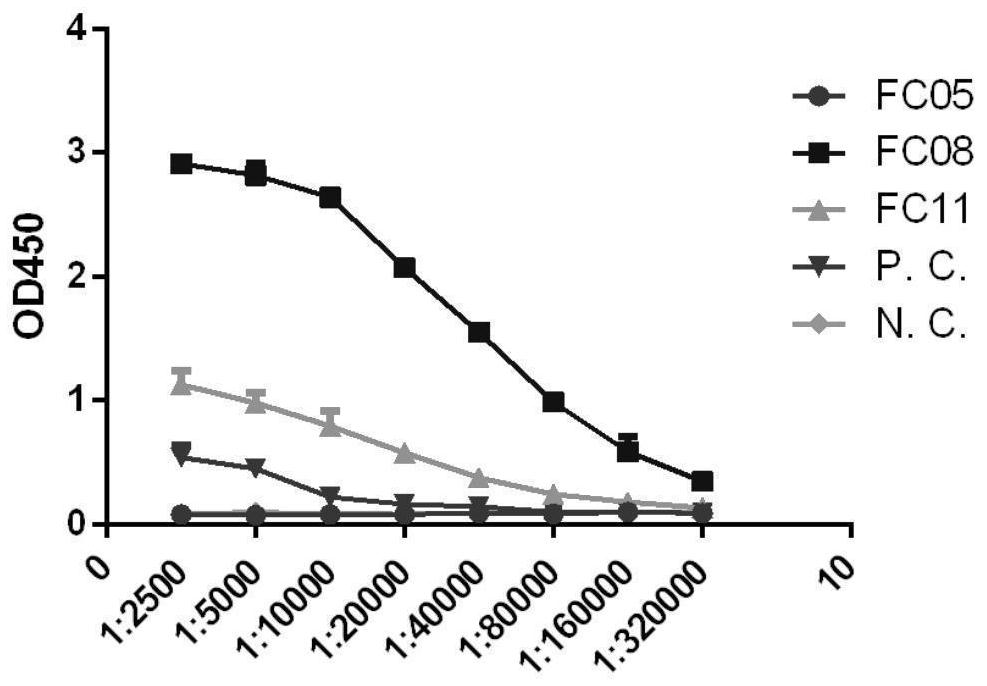
[0130] Example 2 Indirect ELISA detection antibody binding specificity to S-RBD and S-ECD
[0131] Three human antibodies were selected from the 159 antibodies obtained through screening, and they were constructed into IgG-form human full-molecular antibodies (the three antibodies were named FC05, FC08, and FC11), expressed in 293F cells, and purified with Protein A Backup.
[0132] The FC05 antibody sequence is as follows:
[0133] The CDR1 sequence of the heavy chain variable region is shown in SEQ ID NO.1, the sequence of the CDR2 of the heavy chain variable region is shown in SEQ ID NO.2, and the CDR3 sequence of the heavy chain variable region is shown in SEQ ID NO.3; The CDR1 sequence of the light chain variable region is shown in SEQ ID NO.5, the CDR2 sequence of the light chain variable region is shown in SEQ ID NO.6, and the CDR3 sequence of the light chain variable region is shown in SEQ ID NO.7. The amino acid sequence of the heavy chain variable region is shown i...
Embodiment 3
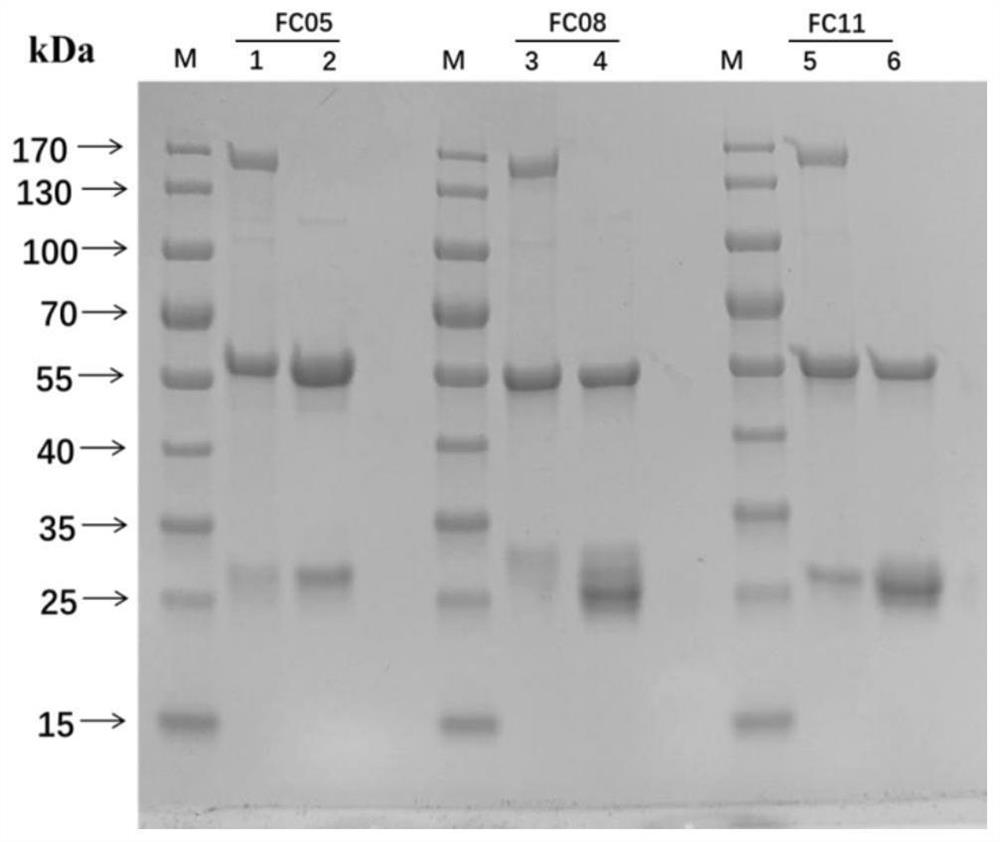
[0138] Embodiment 3 Immunoprecipitation experiment of antibody, S-RBD and S-ECD
[0139] In the early stage, Western Blot was used to detect the binding specificity of the three antibodies to S-RBD and S-ECD, and it was found that none of the three antibodies reacted with S-RBD and S-ECD after SDS-PAGE, indicating that the three antibodies were all conformational epitope. Therefore, the immunoprecipitation (Immunoprecipitation, IP) method was used to detect the binding specificity of the antibody to the target protein, as follows:
[0140] Combine FC05, FC08 and FC11 triclonal antibodies with 20μL Protein A beads at room temperature for 2min, then wash away unbound antibodies with 20mM sodium phosphate. Then 20 μg of target antigens (S-RBD and S-ECD) were added to the antibody and Protein A gel mixture, and combined at room temperature for 2 minutes. Wash away the unbound antigen with 20mM sodium phosphate, elute the antigen-antibody complex with 30μl Gly-HCl buffer (pH 3.0)...
PUM
| Property | Measurement | Unit |
|---|---|---|
| affinity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com