A kind of polysubstituted oxazoline compound and preparation method thereof
An oxazoline compound and multi-substitution technology, applied in the direction of organic chemistry, can solve the problems of cumbersome raw material synthesis steps, poor functional group tolerance, harsh reaction conditions, etc. The effect of mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
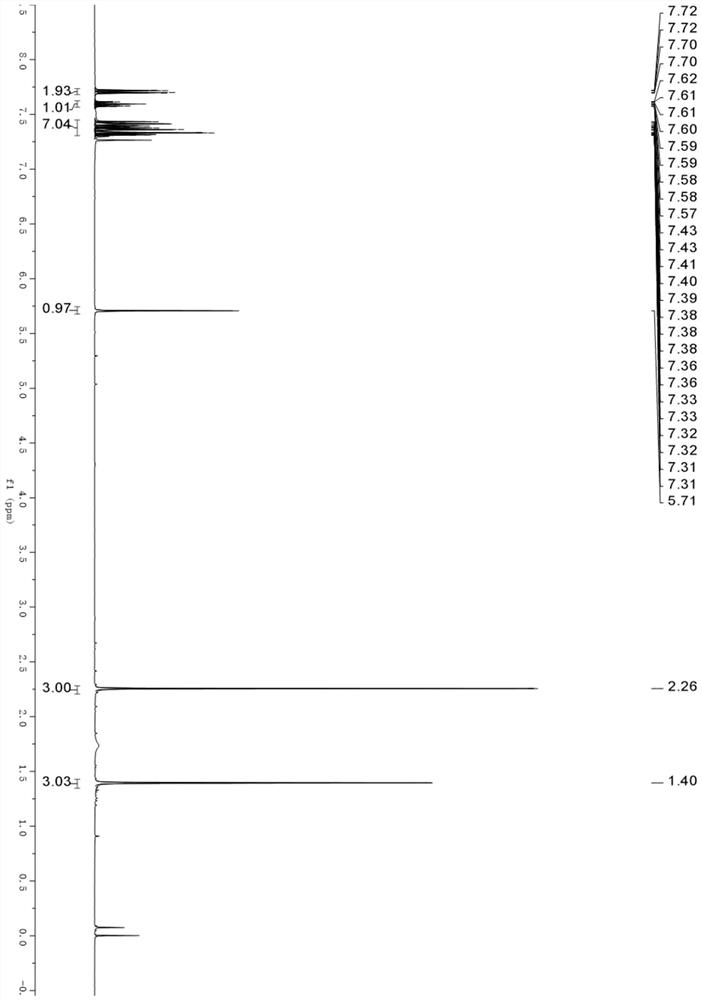
[0031] N-(1-phenylvinyl)acetamide (0.225 mmol, 36.3 mg), benzoylthioylide (0.15 mmol, 29.4 mg), and silver bis-trifluoromethanesulfonimide were added to the 4 mL reaction flask. Salt (0.015 mmol, 5.8 mg), pivalic acid (0.15 mmol, 15.3 mg) and 1 mL of trifluorotoluene; nitrogen was blown into the reaction flask for 30 s, and heated and stirred, and heated to 100 ° C, and the stirring time was 24 h; After the reaction, the reaction solution was cooled to room temperature without post-treatment, and was separated by silica gel column chromatography to obtain a pure product as a yellow solid; the yield was 85% (1.9:1).
[0032] The H NMR data of this product are as follows: 1 HNMR (400MHz, Chloroform-d) δ 7.71 (dd, J=8.4, 1.4Hz, 2H), 7.62–7.57 (m, 1H), 7.45–7.31 (m, 7H), 5.71 (s, 1H), 2.26 (s,3H),1.40(s,3H).
[0033] Thus, the product obtained in the embodiment of the present invention is (trans-2,4-dimethyl-4-phenyl-4,5-dihydrooxazol-5-yl)(phenyl)methanone, and its chemical str...
Embodiment 2
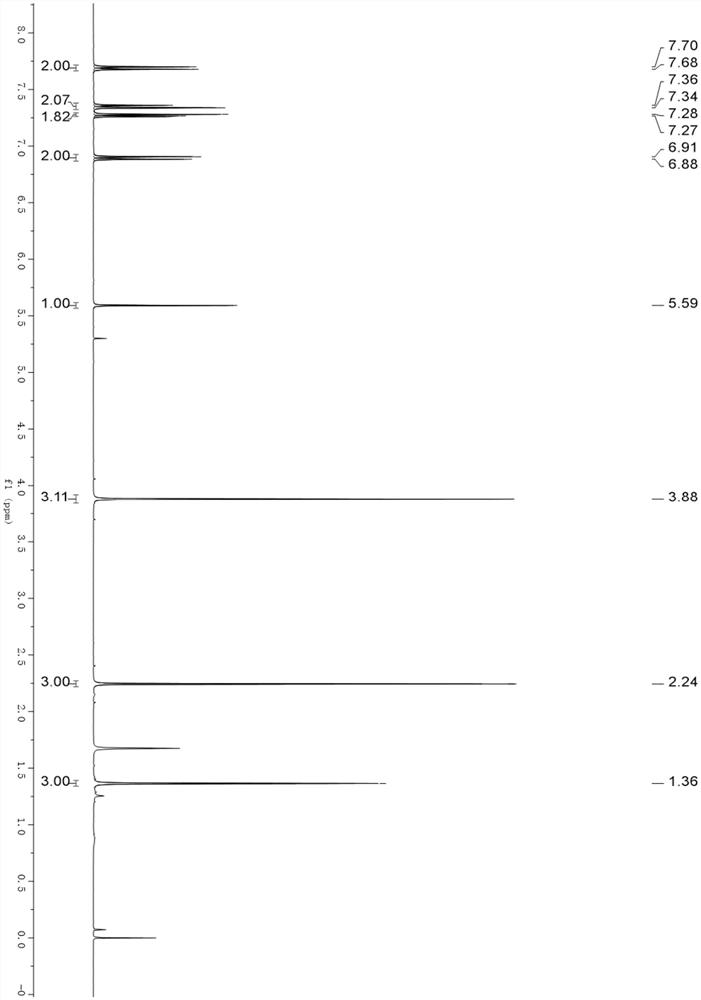
[0036] N-(1-(4-chlorophenyl)vinyl)acetamide (0.225mmol, 44.0mg), benzoylthioylide (0.15mmol, 29.4mg), bistrifluoromethyl were added to the 4mL reaction flask, respectively. Sulfonyl imide silver salt (0.015mmol, 5.8mg), pivalic acid (0.15mmol, 15.3mg) and 1 mL of trifluorotoluene; blow nitrogen into the reaction flask for 30s, heat and stir, and heat to 100°C, The stirring time was 24h; after the reaction, the reaction solution was cooled to room temperature without post-treatment, and the pure product was separated by silica gel column chromatography to obtain a yellow oily product; the yield was 78% (2.1:1).
[0037] The H NMR data of this product are as follows: 1HNMR (400MHz, Chloroform-d) δ 7.71 (dd, J=8.3, 1.3Hz, 2H), 7.64–7.59 (m, 1H), 7.44 (td, J=7.3, 0.9Hz, 2H), 7.36–7.31 (m, 2H), 7.28–7.23(m, 2H), 5.63(s, 1H), 2.25(s, 3H), 1.37(s, 3H).
[0038] Thus, the product obtained in the embodiment of the present invention is (trans-4-(4-chlorophenyl)-2,4-dimethyl-4,5-dihydr...
Embodiment 3
[0041] N-(1-phenylvinyl)propionamide (0.225 mmol, 39.4 mg), benzoylthioylide (0.15 mmol, 29.4 mg), and silver bis-trifluoromethanesulfonimide were added to a 4 mL reaction flask. Salt (0.015 mmol, 5.8 mg), pivalic acid (0.15 mmol, 15.3 mg) and 1 mL of trifluorotoluene; nitrogen was blown into the reaction flask for 30 s, and heated and stirred, and heated to 100 ° C, and the stirring time was 24 h; After the reaction, the reaction solution was cooled to room temperature without post-treatment, and was separated by silica gel column chromatography to obtain a pure product in the form of yellow oil; the yield was 93% (1.7:1).
[0042] The H NMR data of this product are as follows: 1 HNMR (400MHz, Chloroform-d) δ 7.73–7.69 (m, 2H), 7.62–7.56 (m, 1H), 7.40 (dd, J=14.9, 7.6Hz, 3H), 7.36–7.29 (m, 4H) ,5.69(s,1H),2.57(qd,J=7.7,3.2Hz,2H),1.40(s,3H),1.37(d,J=15.0Hz,3H).
[0043] Thus, the product obtained in the embodiment of the present invention is (trans-2-ethyl-4-methyl-4-phenyl-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com