Preparation method of glimepiride tablets
A technology of Glimex and urea tablets, applied in the field of medicine and chemical industry, can solve the problems of poor similarity, achieve stable results, save time, and improve drug dissolution and bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0031] A preparation method of glimepiride tablet, is characterized in that, comprises the following steps:
[0032] S1. Micronize the glimepiride raw material drug by jet milling method, and control the particle size of the glimepiride raw material drug powder to be less than 10 μm;
[0033] S2. Weigh the glimepiride raw material powder and auxiliary materials pulverized in step S1 in proportion. The auxiliary materials include lactose, sodium starch glycolate, microcrystalline cellulose, pregelatinized starch, and magnesium stearate;
[0034] S3. Mix, pulverize and sieve the glimepiride raw material and lactose weighed in step S2, and add part of carboxymethyl starch sodium into a wet granulator for mixing to obtain a premixed powder;
[0035] S4, add povidone to the wet granulator in step S3, make soft material, granulate, fluidized bed drying;
[0036] S5, adding the dried granules to the remaining sodium starch glycolate, pregelatinized starch, and magnesium stearate wei...
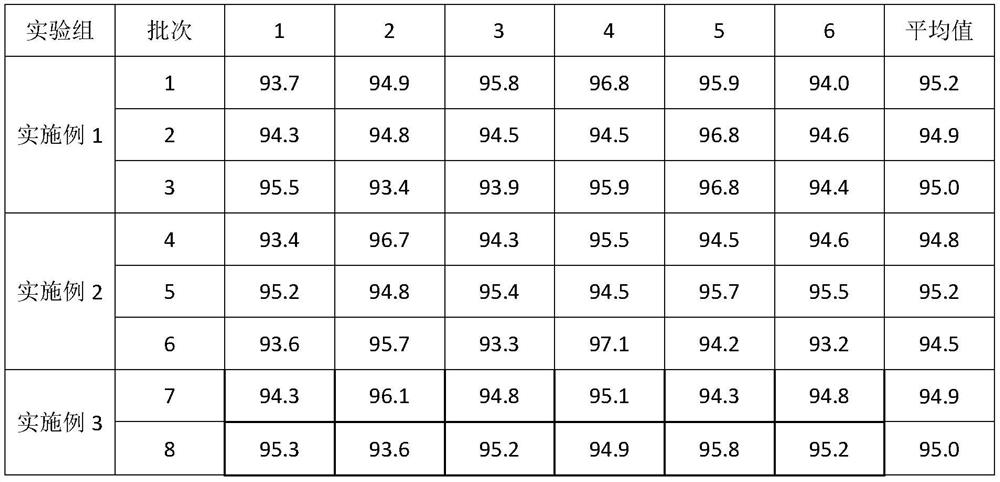
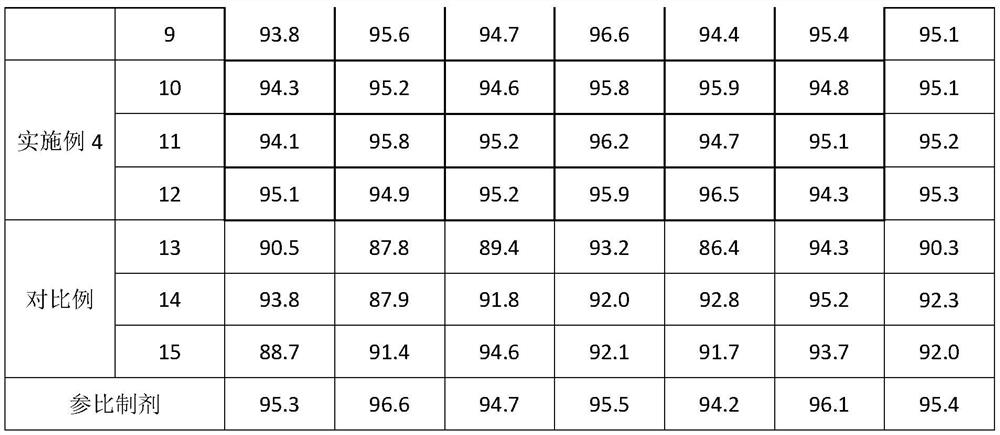
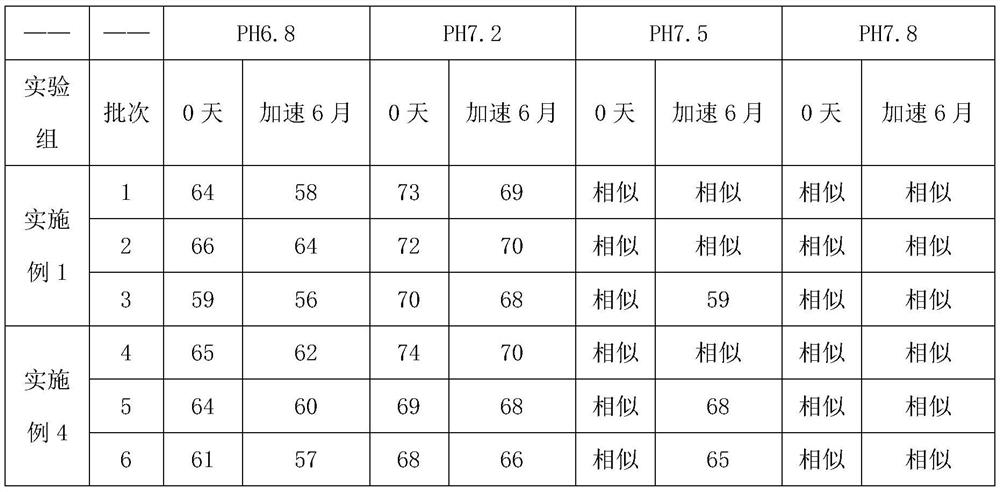
Embodiment 1
[0042] A preparation method of glimepiride tablet, is characterized in that, comprises the following steps:
[0043]S1. Micronize the glimepiride raw material drug by jet milling method, and control the particle size of the glimepiride raw material drug powder to be less than 10 μm;
[0044] S2. Weigh 2kg of glimepiride raw material powder pulverized in step S1, 55kg of lactose, 5.2kg of sodium starch glycolate I, 0.4kg of povidone, 0.25kg of microcrystalline cellulose, and 1.9kg of sodium starch glycolate II in proportion. , magnesium stearate 0.325kg;
[0045] S3, mixing and pulverizing the glimepiride bulk drug and lactose weighed in step S2 through a 100-mesh sieve, and adding it to a wet granulator for mixing with sodium starch glycolate I for 25 minutes to obtain a premixed powder;
[0046] S4, the povidone in step S2 is formulated into 4% povidone aqueous solution, adds 4% povidone aqueous solution (W / W) to the wet granulator in step S3, makes soft material, passes Gr...
Embodiment 2
[0049] A preparation method of glimepiride tablet, is characterized in that, comprises the following steps:
[0050] S1. Micronize the glimepiride raw material drug by jet milling method, and control the particle size of the glimepiride raw material drug powder to be less than 5 μm;
[0051] S2. Weighing 0.7kg of glimepiride bulk drug powder pulverized in step S1, 58kg of lactose, 10kg of sodium starch glycolate, 0.35kg of povidone, 0.06kg of microcrystalline cellulose, 4kg of sodium starch glycolate II, stearin Magnesium acid 0.325kg;
[0052] S3, mixing and pulverizing the glimepiride bulk drug and lactose weighed in step S2 through a 100-mesh sieve, and adding it to a wet granulator for mixing with sodium starch glycolate I for 20 minutes to obtain a premixed powder;
[0053] S4, the povidone in step S2 is formulated into 4% povidone aqueous solution, adds 4% povidone aqueous solution (W / W) to the wet granulator in step S3, makes soft material, passes Granulate with a 20-...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com