A luminescent bacterium freeze-drying protective agent, freeze-dried powder and application of the freeze-dried powder in on-line monitoring of comprehensive toxicity of water quality
A technology of freeze-drying protective agent and luminescent bacteria, which is applied to bacteria, test water, measuring devices, etc., can solve the problems of low formula protection efficiency, unreliable test results, short maintenance period, etc., to improve the survival rate and stabilize the biological activity. performance, reduce the operating cost of reagent consumables, and improve the effect of continuous and stable operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] The bacteria content of the luminescent bacteria prepared by the protective agent of different proportions of embodiment 1
[0031] Cultivate Vibrio fischeri (standard bacterium of luminous bacteria) according to the steps in the "specific application mode" of the present invention, and finally add the protective agent with the values listed in Table 1, freeze-dry, and store at a low temperature in a -80°C refrigerator , resuscitated with 3% sodium chloride when taken out, carried out 10-fold gradient dilution, counted by plate culture, and obtained the number of Vibrio fischeri contained in each different protective agent ratio, and the blank control used 14g of skim milk dissolved in 100mL Protective agent was prepared in sterile water as the control group.
[0032] Table 1
[0033] group Skimmed milk (g) Sucrose (g) Sodium chloride (g) Bacteria content (CFU / g) group 1 8 1 0.5 3.1*10 8 CFU / g
[0034] It can be seen from the results tha...
Embodiment 2
[0035] Determination of the optimal addition amount of embodiment 2 protective agent
[0036] The protective agent formula of Group 3 in Example 1 is used for the production of Vibrio fischeri freeze-dried powder, respectively according to the volume ratio of freeze-dried protective agent 1:1, 1:2, 1:3, 1:4, 1: 5, 1:6, 1:7 (Table 2) were added to the Vibrio fischeri bacteria liquid, and the bacteria content of the freeze-dried powder was measured after adding different amounts of protective agents. The detailed operation steps were the same as in Example 1. At the same time, the bioluminescence tester was used to measure the bacteria content and initial luminescence in different volume ratios.
[0037] Table 2
[0038]
[0039]
[0040] It can be seen from the results that when the volume ratio of the freeze-dried protective agent is 1:5, the prepared freeze-dried powder has a large amount of viable bacteria after resuscitation, and the initial luminescence value is the...
Embodiment 3
[0041] The determination (table 3) of the optimal recovery time of embodiment 3 protective agent
[0042] table 3
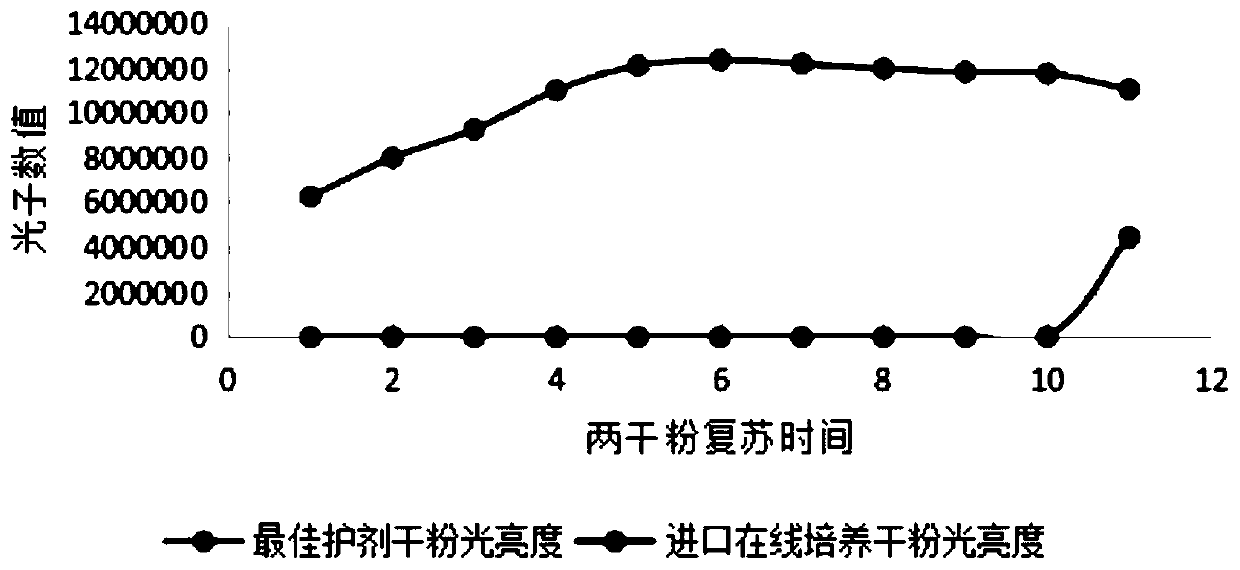
[0043] Freeze-dried powder recovery time Best conditioner dry powder brightness Imported online culture dry powder brightness 1min 6270042 0 2min 8001385 0 3min 9268910 0 4min 11009371 1 5min 12105973 3 10min 12369710 20 20min 12200934 236 30min 11983175 1142 60min 11826740 4967 120min 11760143 15672 1d 11056213 4468207
[0044] Through Table 3 and figure 1 It can be seen that the relative luminous intensity mainly reaches more than 12 million after 5 minutes of resuscitation, and the luminous intensity reaches a steady state within 5-30 minutes. The imported dry powder of online culture needs to be cultivated on-line for 1 day before reaching more than 4 million. Through the comparison of the recovery time and the photon brightness after recovery, it can be concluded tha...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com