A kind of multi-active site fluorescent probe and its synthesis method and application
A technology of active sites and fluorescent probes, applied in the field of analytical chemistry, can solve difficult and difficult problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033]
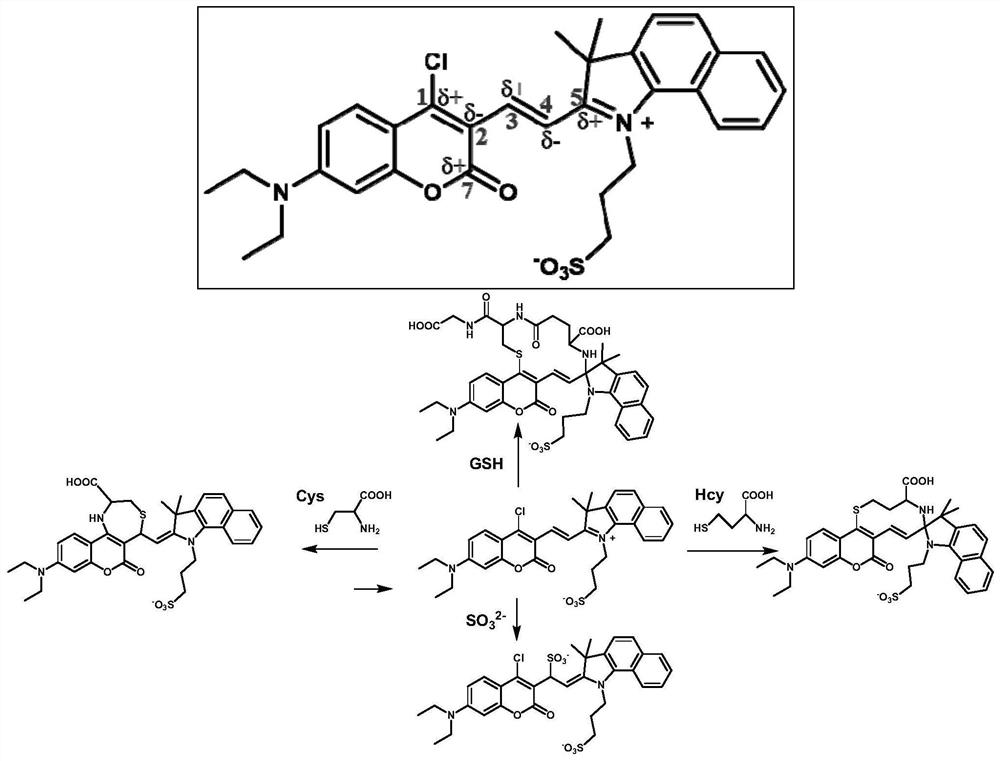
[0034] (E)-3-(2-(2-(4-Chloro-7-(diethylamino)-2-oxo-2H-chromen-3-yl)vinyl)-3,3-dimethyl yl-3H-benzo[g]indol-1-ium-1-yl)propane-1-sulfonate
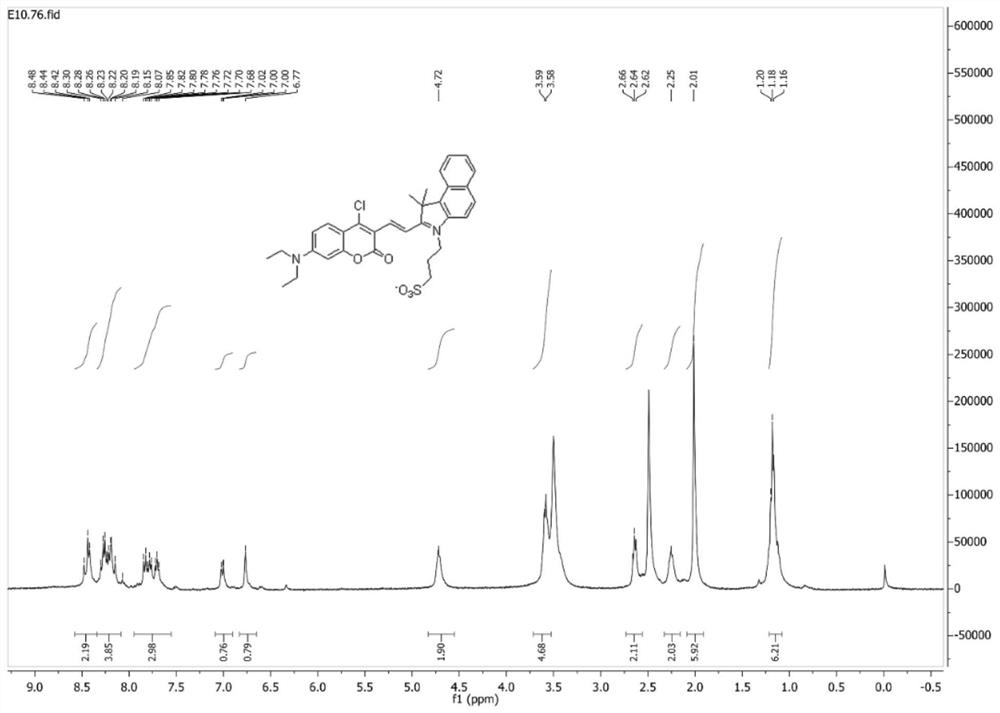
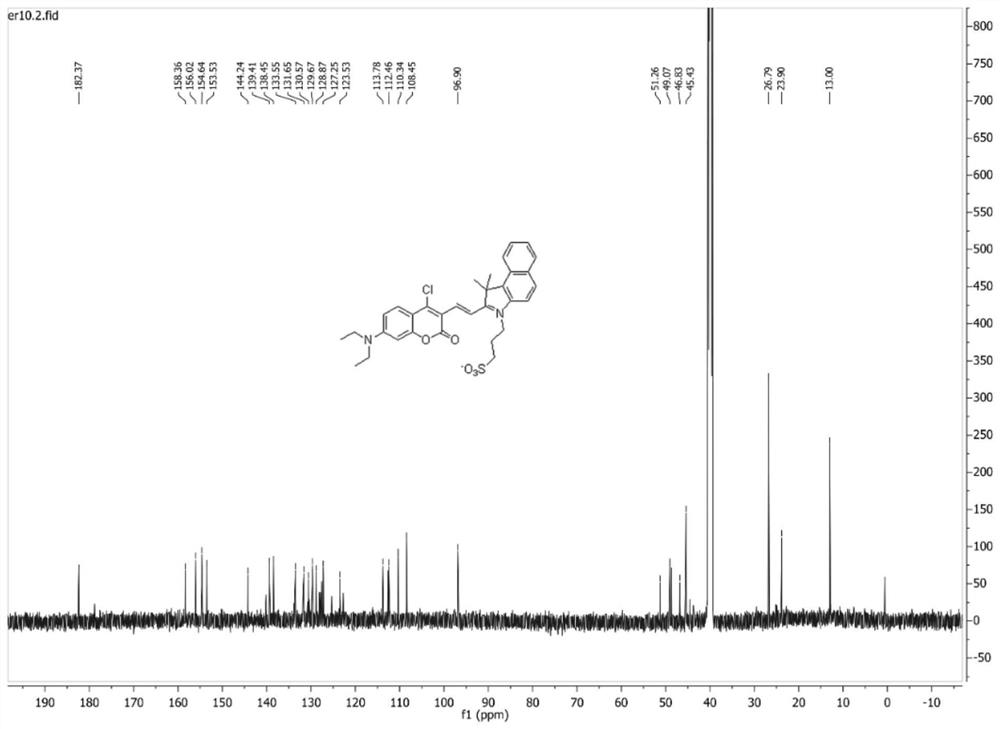
[0035]7-(N,N-diethylamino)-4-chlorocoumarin-3-carbaldehyde (50.0 mg, 179 μmol) and 1,1,2-trimethyl-3-(3-sulfopropyl )-1H-benzo[E]indole internal salt (65.0mg, 197μmol) was dissolved in 10mL of absolute ethanol, 2 drops of acetic acid was added dropwise, under nitrogen protection, the reaction was stirred under reflux; after the reaction, the reaction solution was cooled to room temperature, Concentrated under reduced pressure, purified by silica gel column chromatography (v:v, dichloromethane:methanol=50:1), to obtain 71.0 mg (67% yield) blue-purple solid powder, which was identified as (E)-3-( 2-(2-(4-chloro-7-(diethylamino)-2-oxo-2H-chromen-3-yl)vinyl)-3,3-dimethyl-3H-benzo[ g] Indol-1-ium-1-yl)propane-1-sulfonate.
[0036] Mp 176-179°C. 1 H NMR (300MHz, DMSO-d 6 )δ8.58–8.34(m,2H),8.34–8.08(m,4H),7.95–7.55(m,3H),7.01(d,...
Embodiment 2
[0038] Differential detection of Cys, Hcy, GSH and SO in in vitro environment with a multi-active-site fluorescent probe 2 Applications
[0039] Probes (1 mM) were dissolved in phosphate buffered saline (PBS, 50 mM, pH 7.4, containing 15% v / v acetonitrile) to prepare stock solutions. Solutions of various other analytes were prepared in deionized water. SO 2 The generating reagent is NaHSO 3 . Except for special instructions, the detection conditions of the ultraviolet fluorescence test are: dilute the stock solution with phosphate buffer (50mM, pH 7.4, containing 15% acetonitrile), add the corresponding detection substrate, and the test concentration of the probe is 10μM, at room temperature Incubate for 2 hours, Cys detection uses 365nm wavelength as excitation light, slit is 3nm / 3nm; Hcy detection uses 530nm wavelength as excitation light, slit is 5nm / 5nm; SO2 detection uses 600nm as excitation light, slit is 5nm / 5nm; GSH detection uses 650nm wavelength as the excitatio...
Embodiment 3
[0051] Multi-active site fluorescent molecular probe cytotoxicity test and fluorescence imaging test
[0052] Different biothiols have different levels in cells (Cys is 30-200μM, Hcy is 5-12μM, GSH is 1-10mM). According to relevant reports, the inventors selected human lung cancer cells A549 to conduct biothiol imaging experiments at the cellular level.
[0053] First, the MTT cytotoxicity test of the probe against A549 was carried out. Human lung cancer cells (A549) were grown in DMEM medium containing 10% (v / v) FBS, penicillin (100 U / mL) and streptomycin (100 μg / mL) at 37°C in a humid atmosphere. A549 cells were cultured with different concentrations of the probe (20 μM, 40 μM, 60 μM, 80 μM and 100 μM), and the cell viability results showed that the probe had negligible cytotoxicity to living cells ( Figure 14 ).
[0054] The experimental method for determining the intracellular imaging ability of probes to biothiols is as follows: A-E series first added 1mM N-ethylmalei...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com