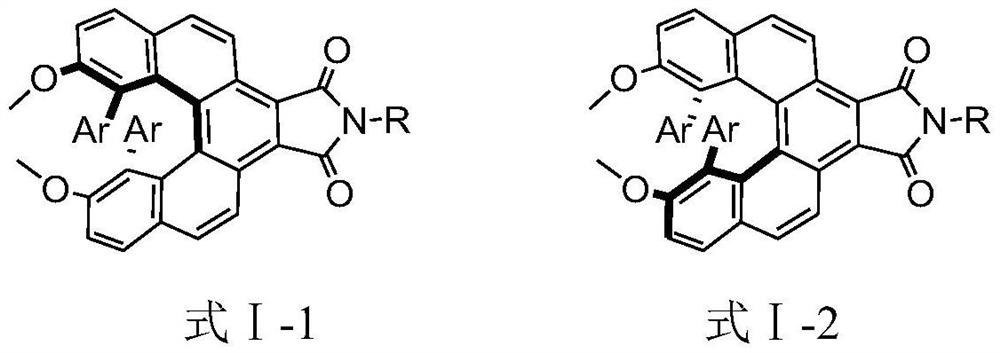
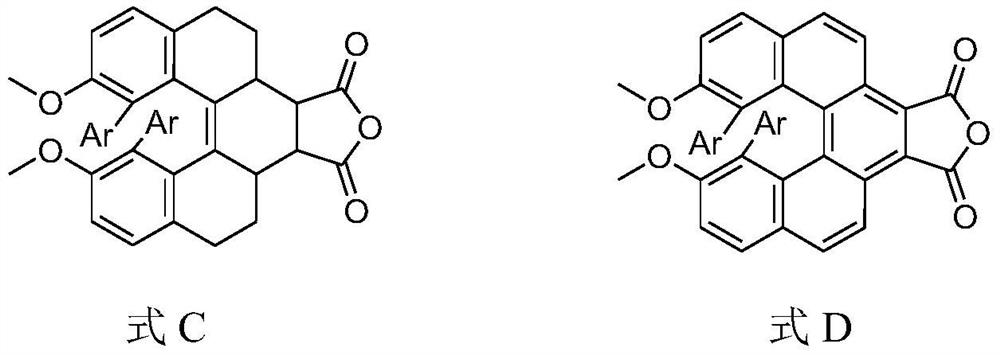
A kind of helicene derivative and its preparation method and application
A technology of derivatives and helicene, which is applied in the field of helicene derivatives and their preparation, can solve problems such as low quantum yield of luminescence, difficulties in preparation and chiral resolution, easy racemization of chirality, etc., and achieve good application prospects, The effect of cheap raw materials and high racemization energy barrier
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] The reaction equation is as follows:
[0045]
[0046] 1) Add 4.75g of A and 3.66g of p-phenylboronic acid (the molar ratio of A to phenylboronic acid is 1:3) into a 250mL two-necked flask, and add 50mL of toluene, 30mL of ethanol and 20mL of 2mol / L Na 2 CO 3 Aqueous solution, add catalyst triphenylphosphine palladium 50mg (5% of the molar weight of A) after ventilating for 5 minutes, reflux for 12 hours, take the organic layer, MgSO 4 Dried, filtered, spin-dried, separated by column chromatography to obtain B 1 3.8g;
[0047] 2) In a 100mL round bottom flask, add 4.7g of B 1 , 4.9g maleic anhydride (B 1 The molar ratio of maleic anhydride to maleic anhydride is 1:5) and 50mL of xylene, heated to reflux for 10 hours, the reaction system was distilled with steam to remove xylene, and the remaining solid was dried, recrystallized with 50mL of acetic anhydride, and filtered to obtain 4.5g of anhydride addition Product C 1 ;
[0048] 3) Add 5.68g C in a 100mL rou...
Embodiment 2
[0057] The reaction equation is as follows:
[0058]
[0059] In the present embodiment 1), 2), 3) and 4) are identical with the reaction steps in " embodiment 1 ".
[0060] The structure detection result of this compound is as follows:
[0061] B 2 :HRMS(APCI)m / z calcd for C 36 h 29 f 6 o 2 [M+H] + 607.2066, found 607.2085.
[0062] C 2 :HRMS(APCI)m / z calcd for C 40 h 31 f 6 o 5 [M+H] + 705.2070, found 705.2075.
[0063] D. 2 :HRMS(APCI)m / z calcd for C 40 h 23 f 6 o 5 [M+H] + 697.1444, found 697.1452.
[0064] E. 2 :HRMS(APCI)m / z calcd for C 46 h 28 f 6 NO 4 [M+H] + 772.1917, found 772.1931.
[0065] From the above detection results, it can be seen that compound B 2 、C 2 、D 2 and E 2 structures are correct.
Embodiment 3
[0067] The reaction equation is as follows:
[0068]
[0069] In the present embodiment 1), 2), 3) and 4) are identical with the reaction steps in " embodiment 1 ".
[0070] The structure detection result of this compound is as follows:
[0071] B 3 :HRMS(APCI)m / z calcd for C 36 h 29 N 2 o 2 [M+H] + 521.2224,found 521.2235.
[0072] C 3 :HRMS(APCI)m / z calcd for C 40 h 31 N 2 o 5 [M+H] + 619.2227, found 619.2236.
[0073] D. 3 :HRMS(APCI)m / z calcd for C 40 h 23 N 2 o 5 [M+H] + 611.1601, found 611.1632.
[0074] E. 3 :HRMS(APCI)m / z calcd for C 46 h 28 N 3 o 4 [M+H] + 686.2074, found 686.2079.
[0075] From the above detection results, it can be seen that compound B 3 、C 3 、D 3 and E 3 structures are correct.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com