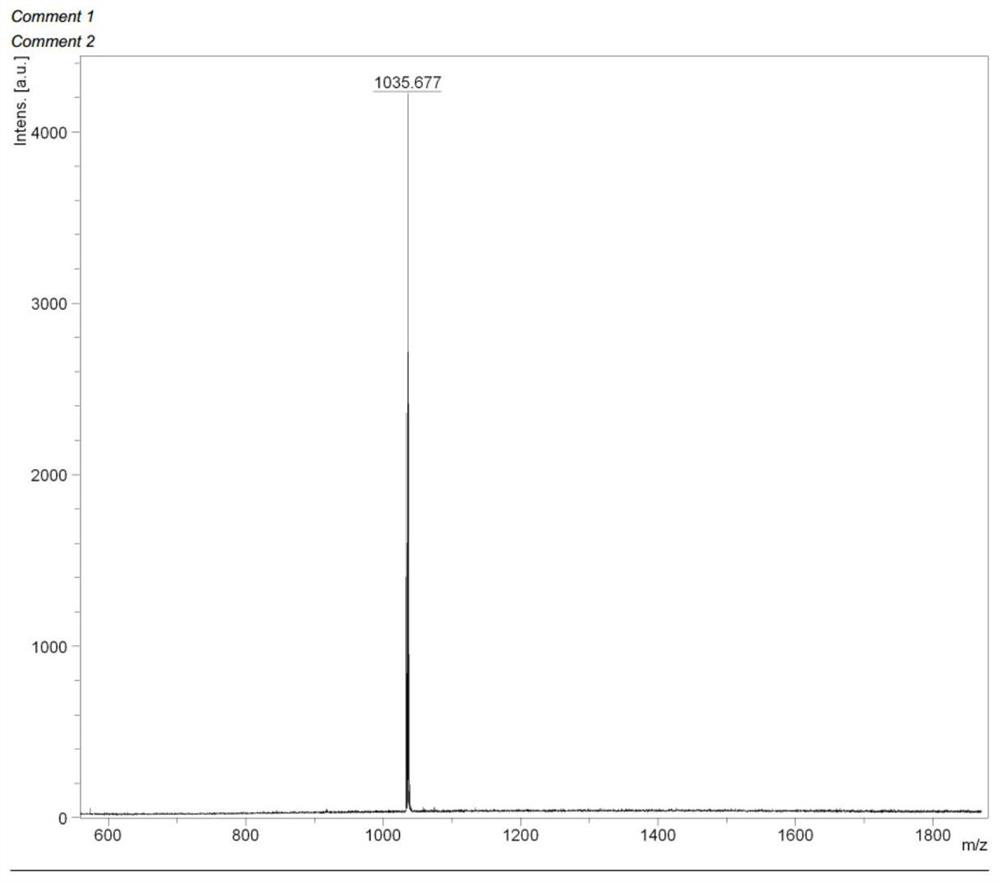
Iridium-coordinated azapyrrolidone photodiagnosis and therapy reagent and its preparation method and application
A diagnosis and treatment reagent, pyrrolidone technology, which is applied in the field of iridium-conjugated azapyrrolidone photodiagnosis and treatment reagents and its preparation, can solve the problems of short absorption wavelength, low penetration depth, unfavorable application at the living body level, etc., and achieve weakening of damage, simple process, good The effect of photothermal and photodynamic effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment 1: Preparation of azapyrrolidone auxiliary ligand
[0041]
[0042] Preparation of Compound 2: Compound 1 (26mmol) and benzaldehyde (26mmol) were dissolved in methanol solution, then 50% NaOH aqueous solution was added, and after stirring and reacting at room temperature for 22 hours, the pH was adjusted to neutrality, and the methanol solvent was removed by filtration to obtain Pale yellow solid powder, then washed three times with water to remove the salt to give the white product 2. Yield: 91%.
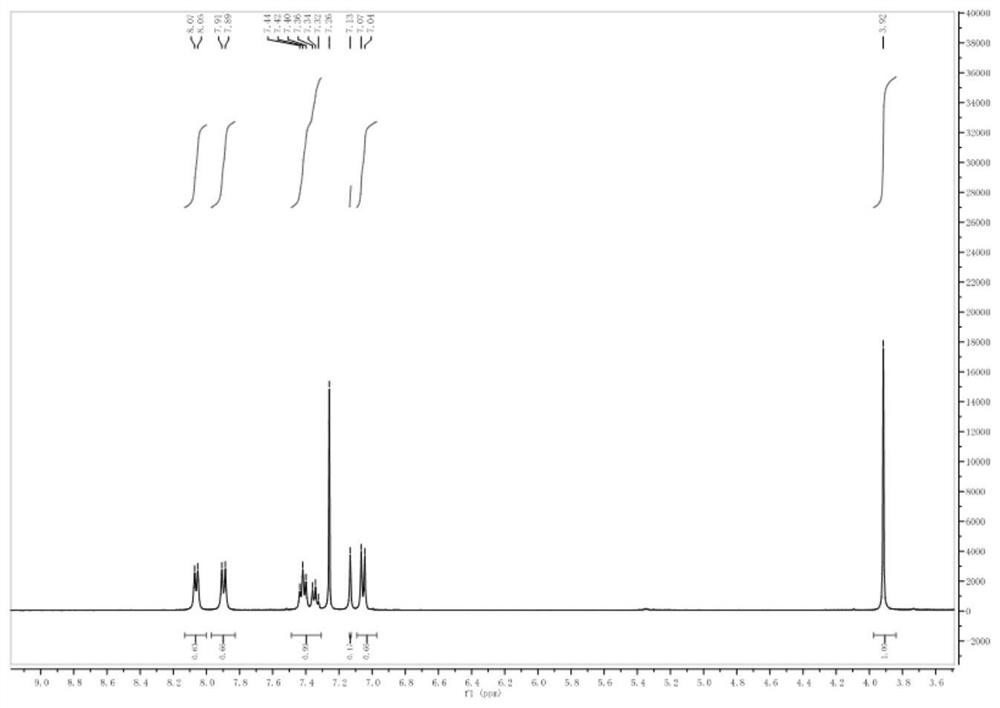
[0043] 1 H NMR (400MHz, CDCl 3 )δ (ppm): 8.01 (d, J = 8.4Hz, 2H), 7.78 (d, J = 16.4Hz, 1H), 7.53 (d, J = 8.4Hz, 2H), 7.35 (d, J = 16.4Hz ,2H),6.98(d,J=8.4Hz,2H),6.69(d,J=8.4Hz,2H),3.77-3.65(s,6H). 13 C NMR (100MHz, CDCl 3 )δ (ppm): 188.85, 169.10, 162.20, 151.91, 144.95, 131.97, 130.49, 130.27, 122.82, 116.63, 114.27.
[0044] The preparation of compound 3: in the methanol solution of compound 2 (5mmol), add 10mL diethylamine and 15mL nitromethane, after ...
Embodiment 2
[0049] Embodiment 2: Preparation of iridium coordination azapyrrolidone complex
[0050]
[0051] The preparation of compound 7: compound 6 (0.14mmol) and iridium trichloride trihydrate are dissolved in the mixed solution of ethylene glycol ether and water, under N 2 Stir overnight at 110°C under atmosphere, then add water to the reaction solution, at this time, a large amount of yellow solid precipitates, the solution is removed by suction filtration, and then washed repeatedly with ethanol to obtain a yellow solid 7.
[0052] Preparation of Compound 8: Compound 7 (0.10 mmol) and silver trifluoromethanesulfonate were dissolved in an aqueous ethanol solution, and refluxed at 100° C. for 24 h. Subsequently, the reaction solution was removed, the obtained solid was dissolved in dichloromethane, the filter residue was removed by filtration, and the filtrate was spin-dried to obtain a yellow solid 8.
[0053] Preparation of compound IrDipy-7: Compound 5 was dissolved in tetrah...
Embodiment 3
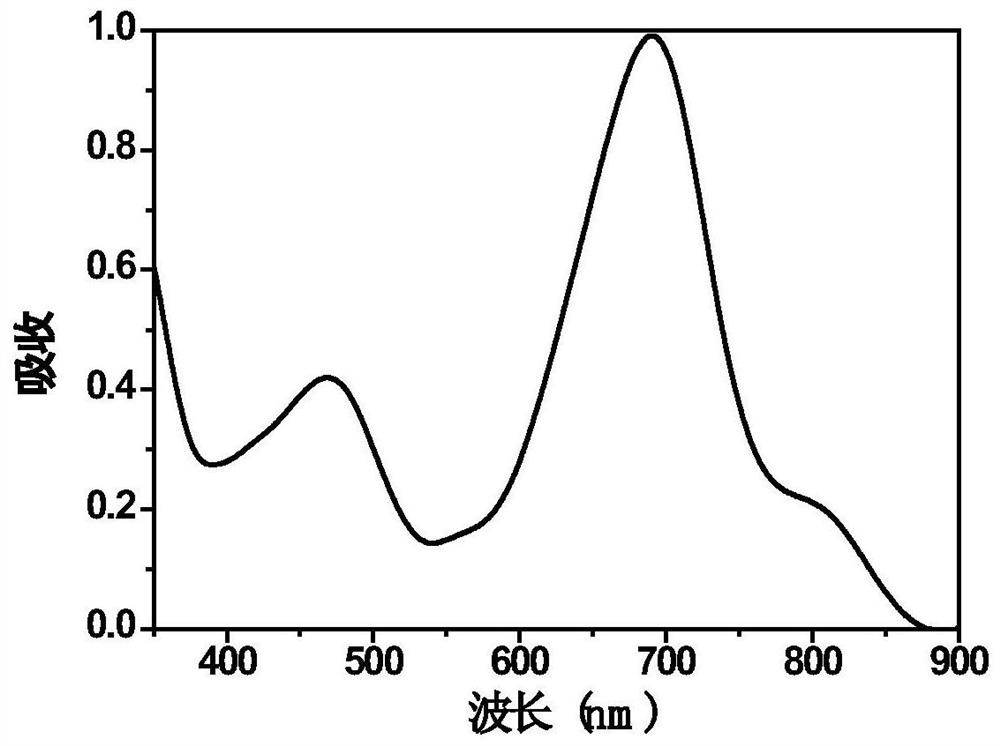
[0055] Embodiment 3: Absorption and emission spectrum test of iridium complex IrDipy-7
[0056] The spectrum test concentration used is 10μM, and the test solvent is CH 2 Cl 2 solution, when measuring the emission spectrum, the excitation wavelength is 690nm.
[0057] Absorption and emission spectra of IrDipy-7 as image 3 with Figure 4 shown. The complex shows strong absorption at 400-500nm in the ultraviolet region and 600-800nm in the near-infrared region. In particular, the complex can be excited by near-infrared light, which greatly reduces the impact of the excitation light source on the cells when doing cell imaging experiments. damage.
[0058] Its emission is wide, and the emission peak is located at 730nm. Red light emission increases the penetration depth of biological tissue, making it more suitable for biological imaging.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com