Dentifrice comprising zinc - amino acid complex
A technology of complexes and amino acids, used in medical preparations containing active ingredients, dentistry, oral care, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0150] The preparation of embodiment 1-ZLC
[0151] The general reaction that forms ZLC in the present embodiment is as follows:
[0152] ZnO+2(lysine·HCl)-? [Zn(Lysine) 2 Cl]Cl H 2 O(ZLC)
[0153] A 2:1 molar ratio ZnO:Lysine·HCl suspension was prepared with stirring at room temperature for about 12 hours. The mixture was centrifuged. Transfer 1 ml of the suspension to an NMR tube. The NMR tube was then placed in a closed tube filled with ethanol for crystal growth. After one week many colorless cubic crystals formed. The crystal structure of ZLC crystals was determined by single crystal X-ray diffraction. The dimensions of this complex molecule are 1.7nm*7.8nm*4.3nm. In this complex, the Zn cation is coordinated by two lysine ligands, from which the NH 2 The two N atoms of the group and the O atom from the carboxyl group are on the equatorial plane. It displays a distorted square-pyramidal geometry with apex positions occupied by Cl atoms. This novel structure ge...
Embodiment 2
[0155] Example 2 - Dentifrice with ZLC
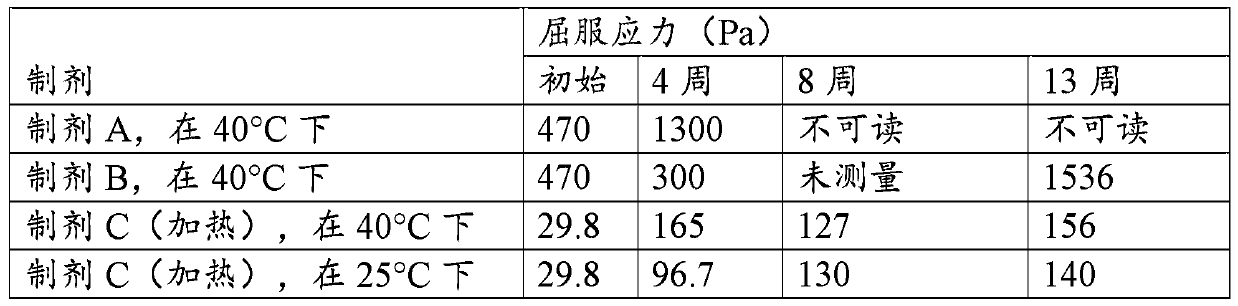
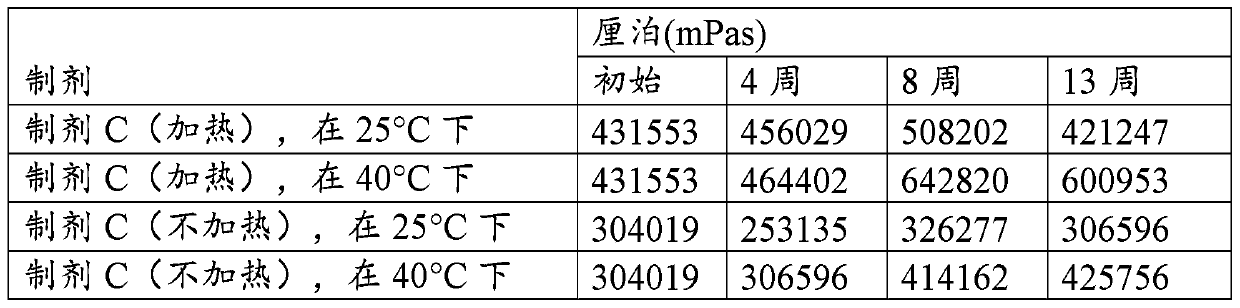
[0156] Dentifrice compositions with ZLC were prepared, the formulations of which are shown in Table 1.
[0157] Table 1
[0158] Composition (weight%) Formulation A Formulation B Formulation C sodium fluoride 0.32 0.32 0.32 Zinc oxide 1.05 1.05 1.05 HCL 37% 0.68 0.68 0.68 Lysine hydrochloride 4.72 4.72 4.72 85% phosphoric acid 0.2 0.2 0 Sodium Hydroxide 50% 0 0 0.5 sodium saccharin 0.8 0.2 0.2 Sucralose 0 0.02 0.02 Titanium dioxide 0.4 0.4 1 CMC sodium type 12 0.2 0.5 0 xanthan gum 0.4 0 0 Plasdone K90 (PVP) 0 1 1 Hydroxyethyl cellulose 0 0 0.2 Surfactant 2.75 2.75 2.75 silica 21 19 26 glycerin 42.28 43.5 36.1 Polyethylene glycol 600 3 3 3 Propylene Glycol 4 4 4 STPP 2 2 2 flavoring agent 1.2 1.7 1.45 Deionized water 15 15 15 pH of the formulation 7.6...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com