Novel coumarin conjugated heterocyclic fluorescent probe for detecting Fe (III)
A coumarin-based, fluorescent probe technology, used in fluorescence/phosphorescence, luminescent materials, measuring devices, etc., can solve the problems of small Stokes shift, high detection limit, low college specificity, etc., and it is easy to achieve reaction conditions. Controlled, high yield, and stable fluorescence properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] (1) Synthesis of 3-bromo-7-diethylaminocoumarin
[0038] Dissolve 2g of 7-diethylaminocoumarin (9.2mmol) in 20mL of glacial acetic acid, stir the solution at room temperature to turn reddish brown, then add 500μL (9.7mmol) of liquid bromine, and react at room temperature for 6h. The reaction was monitored by TLC. After the reaction was complete, part of the solvent was removed under reduced pressure, and then an appropriate amount of acetonitrile was added to the solution to precipitate a white solid, which was suction filtered, washed, and dried. The crude product was purified by column chromatography (dichloromethane / petroleum ether 1:20) to obtain 2.4 g of the product 7-diethylaminocoumarin (yield 88.6%).
[0039] (2) Synthesis of 3-(2-(trimethylsilyl)ethynyl)-1,10-phenanthroline
[0040] Take 2.6g 3-bromo-1,10-phenanthroline (0.01mol), 23.6mg palladium chloride (0.13mmol), 25mg cuprous iodide (0.13mmol), 68.7mg triphenylphosphine (0.26mmol) Dissolve in appropriate...
Embodiment 2
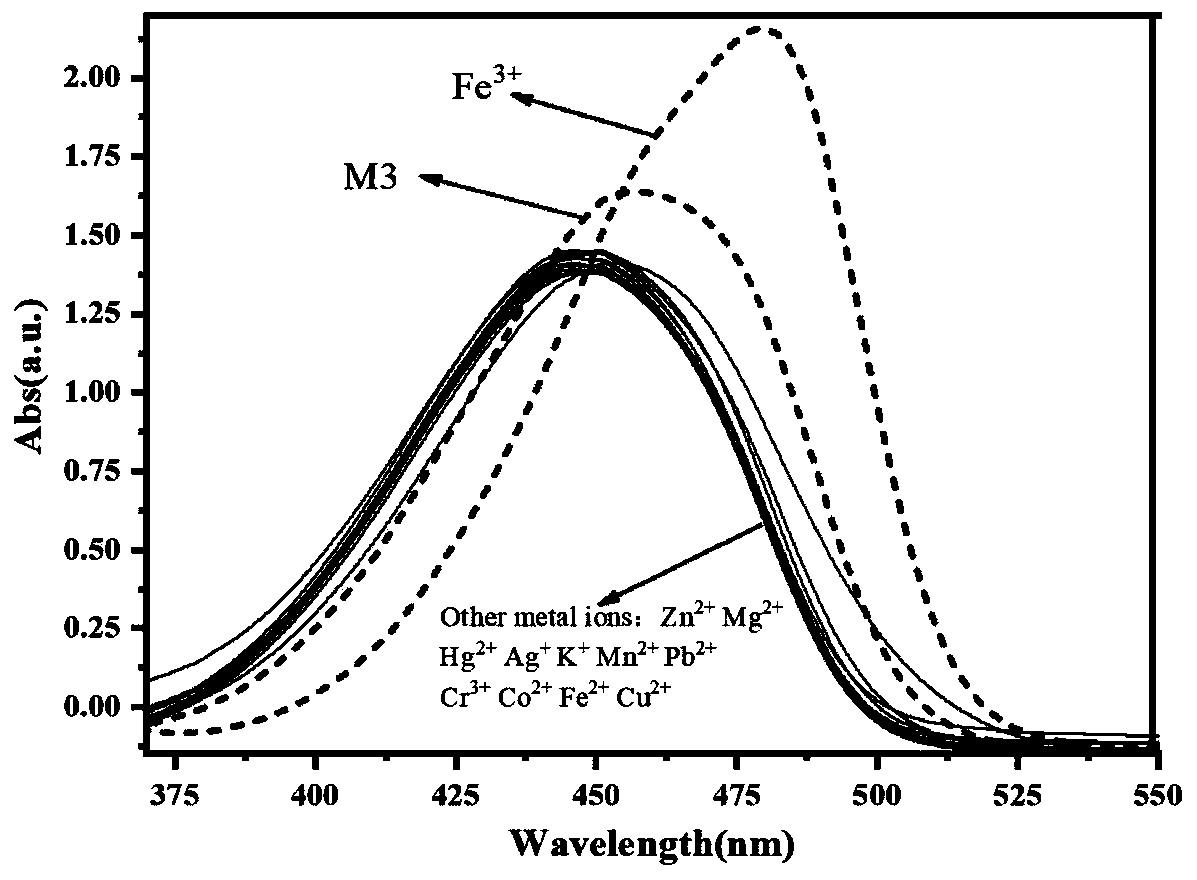
[0048] (1) Probe M3 ion recognition UV spectrum
[0049] Such as figure 1 As shown, it can be known that compound M3(10 -5 mol / L, with absolute ethanol as the solvent) the maximum absorption wavelength is 455nm. When M3 is mixed with other metal ions in an equivalent ratio of 1:1, only Fe 3+ The maximum absorption wavelength of the M3 solution is caused to shift right to 480nm, and the absorbance is obviously enhanced at the same time. At the same time, we use ion interference experiments to detect whether other metal ions will affect the M3 on Fe 3+ recognition, such as figure 2 As shown, the addition of other metal ions does not affect the M3-Fe 3+ The red shift of the mixed solution. This is consistent with the ion recognition pre-experimental results.
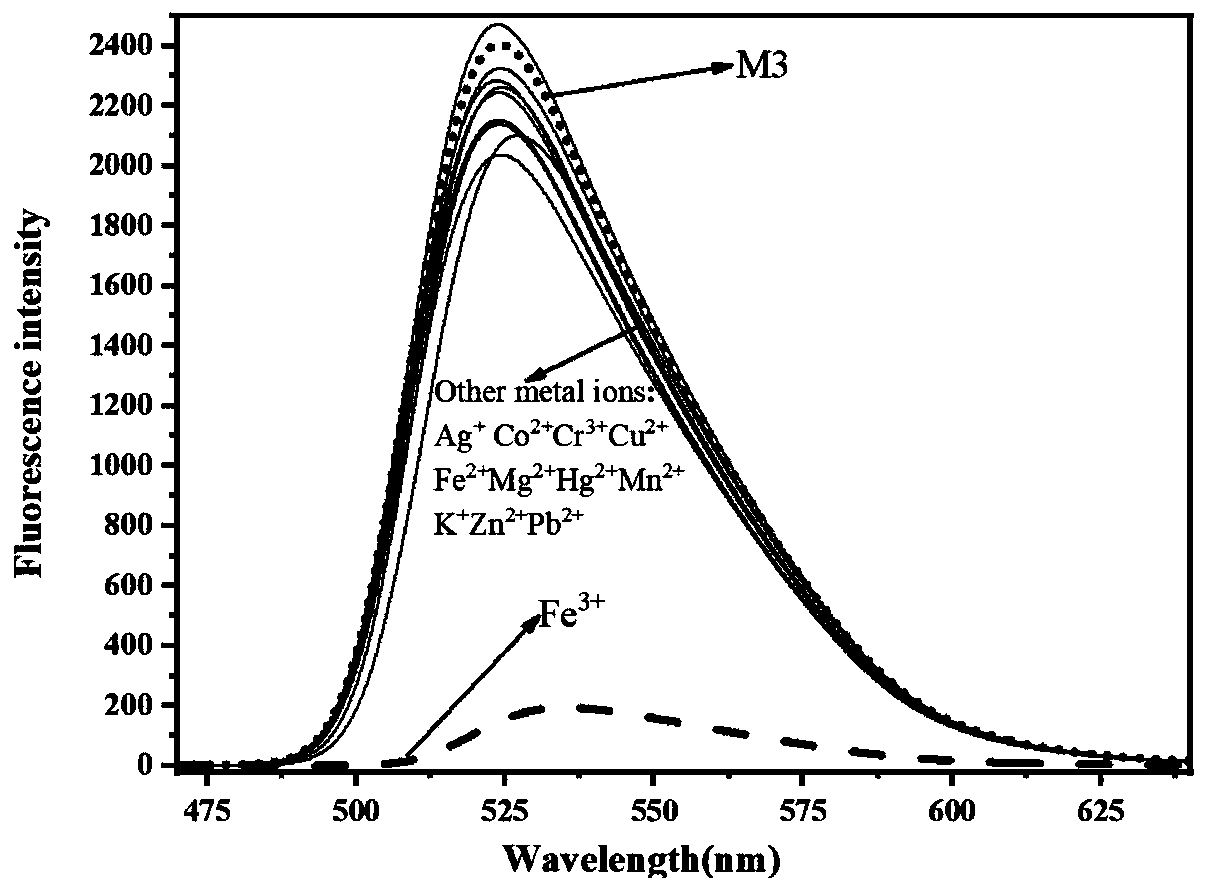
[0050] (2) Probe M3 ion recognition fluorescence spectrum
[0051] 10 with absolute ethanol as solvent -5 The metal ions of mol / L M3 were mixed according to the equivalent ratio of 1:1, and stood still for 30s; th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorption wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com