Sufentanil pharmaceutical composition for transdermal administration as well as preparation method and application of composition
A technology for transdermal drug delivery and composition, which is applied in the field of sufentanil transdermal drug drug composition and its preparation, and can solve the problems of inconvenient drug use and poor compliance of patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
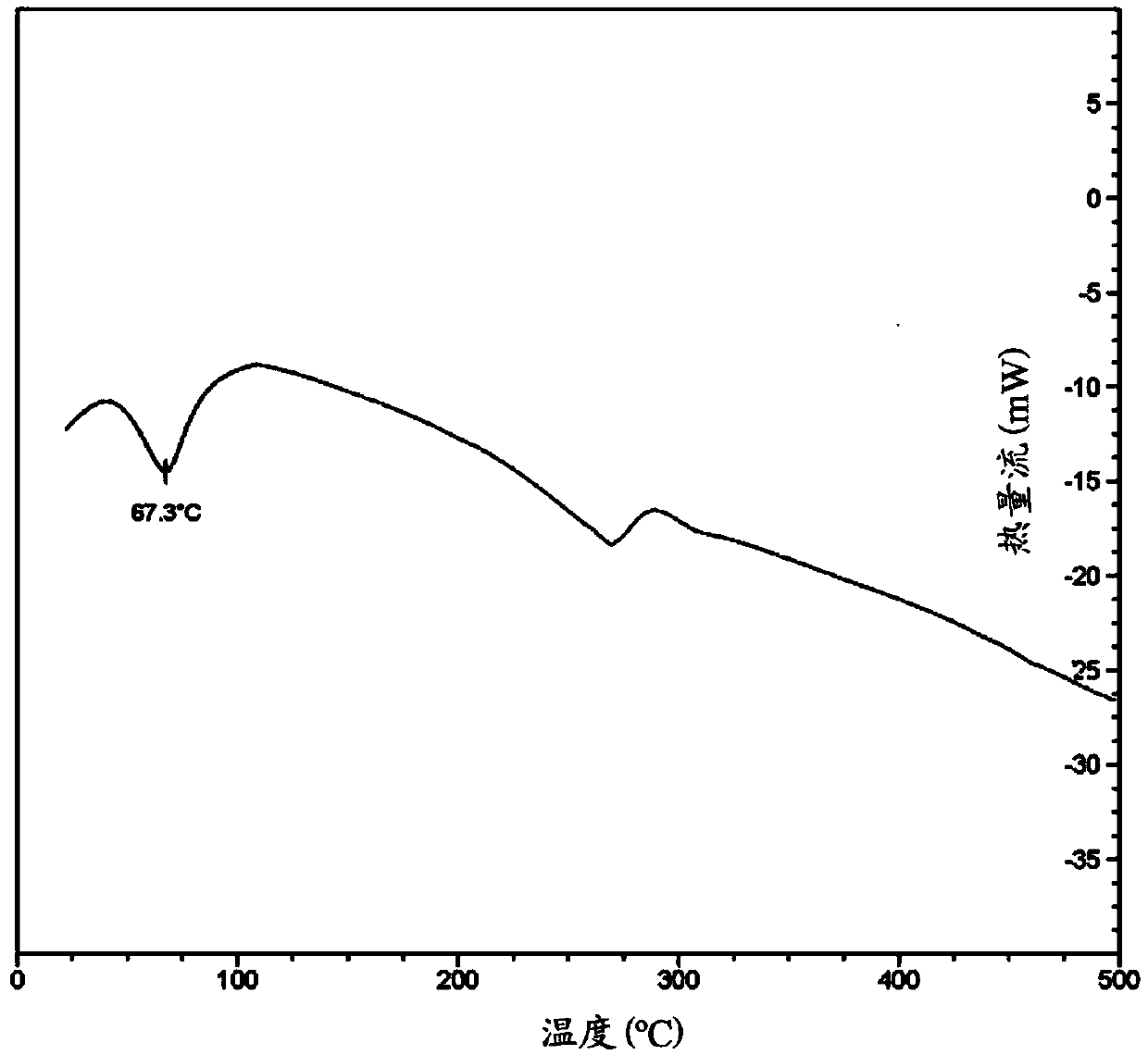
Image
Examples
Embodiment 1
[0067] The transdermal patch preparation of embodiment 1 sufentanil citrate
[0068] (1) Sufentanil citrate 5g, Soluplus 2g, and magnesium stearate 0.1g are micronized, mixed uniformly to obtain a physical mixture, and polyethylene glycol (molecular weight 2000) 2.9g is added;
[0069] (2) Set the extrusion temperature of the twin-screw extruder to 120°C, start the screw after rising to the set temperature, add the physical mixture in step (1) to the extruder, heat-melt and extrude, It is extruded in the form of spherical particles to obtain amorphous particles, which are then micronized, and the particle size is controlled at about 100-150nm.
[0070] (3) Weighing 0.5 g of micronized amorphous particles prepared in step (2), 0.2 g of soybean lecithin, 0.03 g of cholesterol, 3 g of absolute ethanol, and 6.27 g of water;
[0071] (4) Dissolving soybean lecithin, cholesterol, and sufentanil citrate particles in absolute ethanol, heating to 30-45°C to dissolve them to obtain a m...
Embodiment 2
[0075] The transdermal patch preparation of embodiment 2 sufentanil hydrochloride
[0076] (1) Micronize sufentanil hydrochloride 3g, povidone (PVP-S630) 1.5g, and talcum powder 0.2g, mix uniformly to obtain a physical mixture, add polyethylene glycol (molecular weight 3000) 5.3g;
[0077] (2) Set the extrusion temperature of the twin-screw extruder to 100°C, start the screw after rising to the set temperature, add the physical mixture in step (1) to the extruder, heat-melt and extrude, It is extruded in the form of spherical particles to obtain amorphous particles, which are then micronized, and the particle size is controlled at about 150-200nm.
[0078] (3) Weigh 0.3 g of micronized amorphous particles prepared in step (2), 0.2 g of phosphatidylcholine, 0.08 g of cholesterol, 3 g of propylene glycol, and 6.42 g of water;
[0079](4) Dissolving phosphatidylcholine, cholesterol, and sufentanil hydrochloride microparticles in propylene glycol, and heating to 30-45° C. to diss...
Embodiment 3
[0083] The transdermal patch preparation of embodiment 3 sufentanil tosylate
[0084] (1) Micronize sufentanil tosylate 0.5g, povidone (PVP-VA64) 4g, and magnesium stearate 0.3g, mix uniformly to obtain a physical mixture, add polyethylene glycol (molecular weight 4000) 5.2g;
[0085] (2) Set the extrusion temperature of the twin-screw extruder to 140°C, start the screw after rising to the set temperature, add the physical mixture in step (1) to the extruder, heat-melt and extrude, It is extruded in the form of spherical particles to obtain amorphous particles, which are then micronized, and the particle size is controlled at about 250-300nm.
[0086] (3) Weighing 0.5 g of micronized amorphous particles prepared in step (2), 2 g of dipalmitoylphosphatidylcholine, 0.3 g of cholesterol, 30 g of absolute ethanol, and 60.7 g of water;
[0087] (4) dissolving dipalmitoylphosphatidylcholine, cholesterol, and sufentanil tosylate microparticles in absolute ethanol, and heating to 30...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com