Novel drug-loaded microsphere and preparation method thereof
A new technology of drug-loaded microspheres, applied in the preparation of microspheres, pharmaceutical formulations, microcapsule preparations, etc., can solve the problem of reducing the encapsulation efficiency, poor controlled release ability of the microsphere skeleton, and inapplicable to temperature-sensitive protein and polypeptide microspheres. and other problems, to achieve the effect of good adhesion and transfer, good biocompatibility, and easy industrial application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
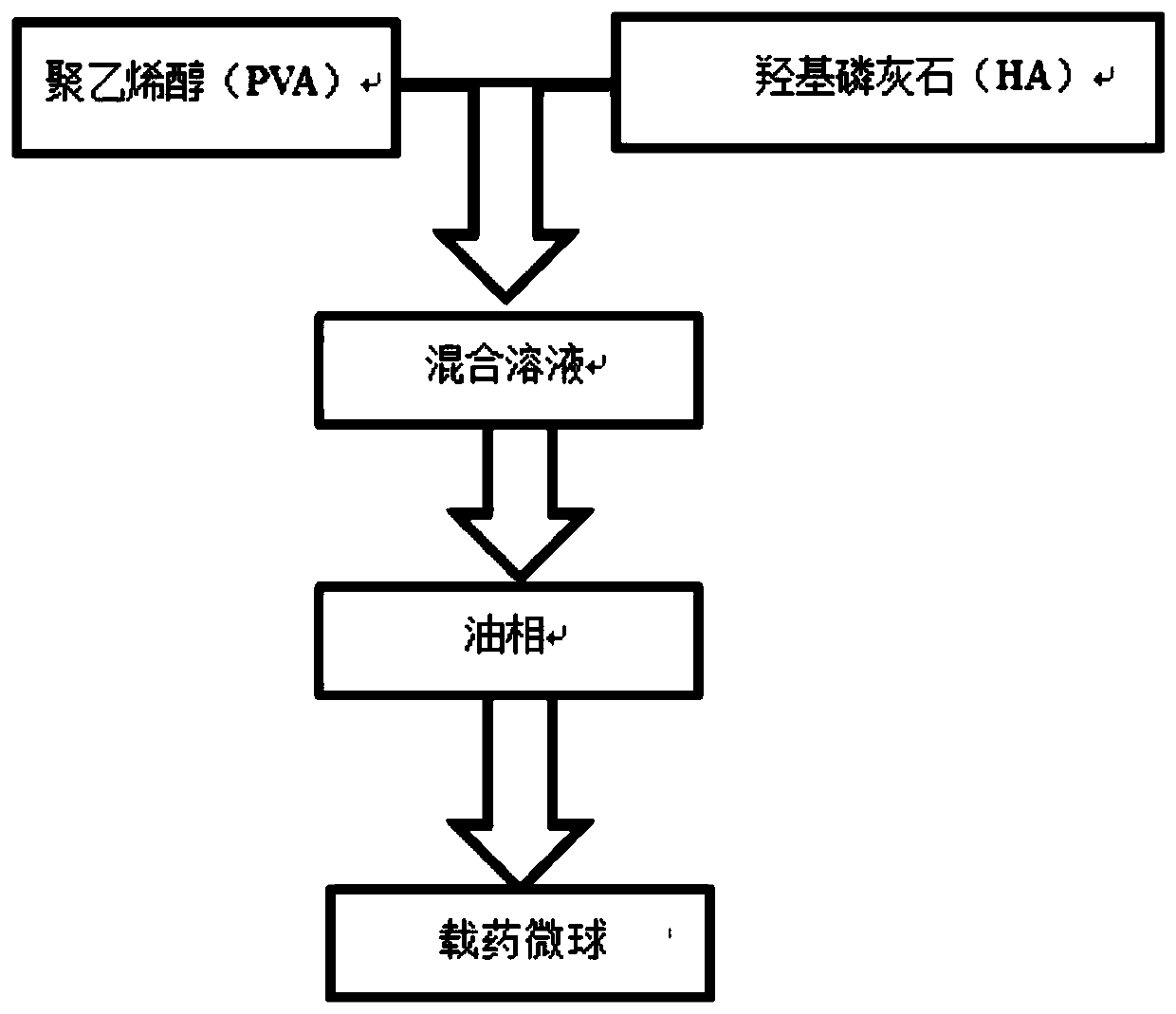
[0045] The preparation method of the novel drug-loadable microspheres of the present invention, as attached image 3 Shown, described preparation method comprises the steps:
[0046] (1) Mix the aqueous solution of polyvinyl alcohol and hydroxyapatite particles evenly; wherein the mass ratio of polyvinyl alcohol to water is 1:100;
[0047] (2) Add concentrated hydrochloric acid in the mixed solution mixed uniformly in step (1), the mass ratio of concentrated hydrochloric acid and polyvinyl alcohol is 14:100-15:100, then add glutaraldehyde, obtain pre-reaction liquid, pre-reaction After 3 seconds, add the pre-reaction liquid dropwise to the mixed oil phase before the pre-reaction liquid condenses, and stir in an oil bath at 50°C-60°C to continue the reaction for at least 3 hours; the mixed oil phase includes liquid paraffin and dehydration Sorbitan fatty acid ester;
[0048] (3) collecting the reacted mixture in step (2) and filtering, washing, and freeze-drying to obtain dru...
Embodiment 2
[0051] An embodiment of the novel drug-loadable microspheres of the present invention, the drug-loadable microspheres described in this embodiment are prepared by the following method:
[0052] (1) Mix the aqueous solution of polyvinyl alcohol and hydroxyapatite particles evenly; wherein the mass ratio of polyvinyl alcohol to water is 2:100;
[0053] (2) Add concentrated hydrochloric acid to the uniformly mixed solution in step (1), the mass ratio of concentrated hydrochloric acid to polyvinyl alcohol is 14:100-15:100. Then add glutaraldehyde to obtain a reaction solution. After pre-reaction for 10 seconds, add the pre-reaction solution dropwise to the mixed oil phase before the pre-reaction solution condenses, and stir in an oil bath at 50°C-60°C to continue the reaction for 4 hours; The mixed oil phase includes liquid paraffin and sorbitan fatty acid ester;
[0054] (3) collecting the reacted mixture in step (2) and filtering, washing, and freeze-drying to obtain drug-loada...
Embodiment 3
[0057] An embodiment of the novel drug-loadable microspheres of the present invention, the drug-loadable microspheres described in this embodiment are prepared by the following method:
[0058] (1) Mix the aqueous solution of polyvinyl alcohol and hydroxyapatite particles evenly; wherein the mass ratio of polyvinyl alcohol to water is 3:100;
[0059] (2) Add concentrated hydrochloric acid to the uniformly mixed solution in step (1), the mass ratio of concentrated hydrochloric acid to polyvinyl alcohol is 14:100-15:100. Then add glutaraldehyde to obtain a reaction solution. After pre-reaction for 30 seconds, add the pre-reaction solution dropwise to the mixed oil phase before the pre-reaction solution condenses, and stir in an oil bath at 50°C-60°C to continue the reaction for at least 3 hours. ; The mixed oil phase includes liquid paraffin and sorbitan fatty acid ester;
[0060] (3) collecting the reacted mixture in step (2) and filtering, washing, and freeze-drying to obtain...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| encapsulation rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com