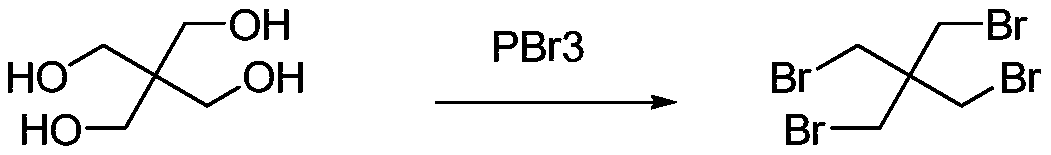
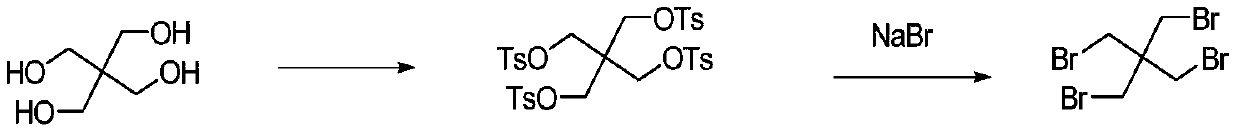
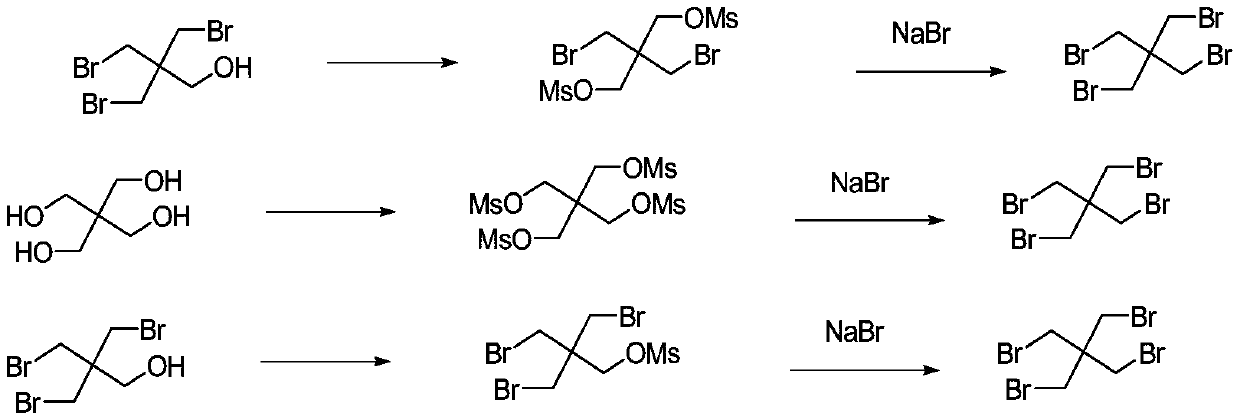
Synthetic method of tetrabromo neopentane
A technology for the synthesis of bromopentane, which is applied in the fields of chemical instruments and methods, preparation of halogenated hydrocarbons, preparation of sulfonate, etc., can solve problems such as easy moisture absorption and complicated feeding process, so as to reduce usage and environment Contamination, effects of mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Add 1.3kg (9.6mol) of pentaerythritol and 6.5L of pyridine into a 20L three-necked flask, and add 5.5L (42mol) of benzenesulfonyl chloride dropwise under stirring. Control the rate of addition so that the reaction temperature is controlled below 35°C, and the drop is completed within 2 hours. After the dropwise addition, continue to stir the reaction at 40°C for 3h until the reaction is complete. After the reaction was completed, the cold bath was cooled to 0°C, and then the above reaction system was added dropwise to 5L of ice water for crystallization. During the dropping process, the temperature was controlled to be lower than 40°C, and a white granular precipitate was formed. After filtering, the filter cake was washed twice with water (5 L×2), once with 5% hydrochloric acid (5 L), and once with methanol (2 L). The filter cake was dried at 70-80° C. to obtain 61.3 g of a white pentaerythritol tetrabenzenesulfonate powder product, with a yield of 92% and a melting p...
Embodiment 2
[0029] Add 1.8L of pyridine into a 5L three-necked flask with magnetic stirring and cool to 0°C, add 593g (2.26mol) of dibromoneopentyl glycol under stirring, and then add p-toluenesulfonyl chloride (1kg) in batches, and the temperature will rise slowly for 20 minutes After the addition, the temperature rose to 20°C, and after the addition, it was moved to room temperature for 12 hours of reaction. The above reaction solution was slowly added dropwise to 2.5L of ice water with stirring, and the temperature was controlled not to exceed 40°C during the dropwise addition, and then filtered, and the filter cake was washed successively with 2N hydrochloric acid (1L), and washed with water until neutral. Then 2L of ethanol was rinsed, and the filter cake was dried to obtain 1314g of product, and the yield was greater than 100%.
[0030] Weigh 4mol of the above-mentioned intermediate, 1266g (3eq) of sodium bromide, and 8.5L of diethylene glycol in a 20L three-necked flask. Add 10L o...
Embodiment 3
[0032] Add 9.6 mol of dibromoneopentyl glycol and 6.5 L of pyridine into a 20 L three-necked flask, and add 21 mol of benzenesulfonyl chloride dropwise under stirring. Control the rate of addition to keep the reaction temperature below 35°C, and drop it in 1.2 hours. After the dropwise addition, the stirring reaction was continued at 30° C. for 4 h. After the reaction was completed, the cold bath was cooled to 2 ° C, and then the above reaction system was added dropwise to 5 L of ice water, and the temperature was controlled to be lower than 40 ° C during the dropping process, and a white granular precipitate was formed. After filtering, the filter cake was washed twice with water (5 L×2), once with 5% hydrochloric acid (5 L), and once with methanol (2 L). The filter cake was dried at 70-80°C to obtain the activated product with a yield of 91.5%.
[0033] Add 12.6mmol of dried activated product, 20ml of diethylene glycol (DEG), 0.75g (1.25mmol) of polyethylene glycol (PEG600...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com