RNA virus inactivation preservation solution and application thereof
A technology of RNA virus and preservation solution, which is applied in the field of virus biological detection, can solve the problems of cumbersome virus decrosslinking process, inactivation of RNA virus, and operator hazards, so as to preserve the integrity of RNA, reduce the risk of infection, and save energy. the effect of time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] (S1) The formula of RNA virus inactivation preservation solution: 2M ammonium chloride, 0.5M 1-ethyl-3-methylimidazolium bisulfate, 0.2M tartaric acid, 0.25M lithium chloride, 20mM ethylenediaminetetraacetic acid , 5 mM ethylene glycol bis(2-aminoethyl ether) tetraacetic acid, 1% (w / v) lithium dodecyl sulfate, 0.5% (w / v) Triton X-100, 0.2% (w / v) Tween 20, 0.05M sodium pyrophosphate, 1% (w / v) dithiothreitol, 50 mM HEPES buffer, pH5.
[0066] The contrast solution used VTM sterile virus transport solution preservation solution (preservation solution prepared by Hank’s solution) (from Shenzhen Huachenyang Technology Co., Ltd.) and Buffer AVL (lysate) in the Viral RNA Mini Kit, the swabs are from Italian COPAN disposable flocking swabs, the pseudovirus (SARS-CoV2) is the quality control substance containing the N gene fragment (Shanghai Yisheng Biotechnology Co., Ltd.), kit use Viral RNA Mini Kit for manual operation. The real-time PCR detection reagents were prepared ...
Embodiment 2
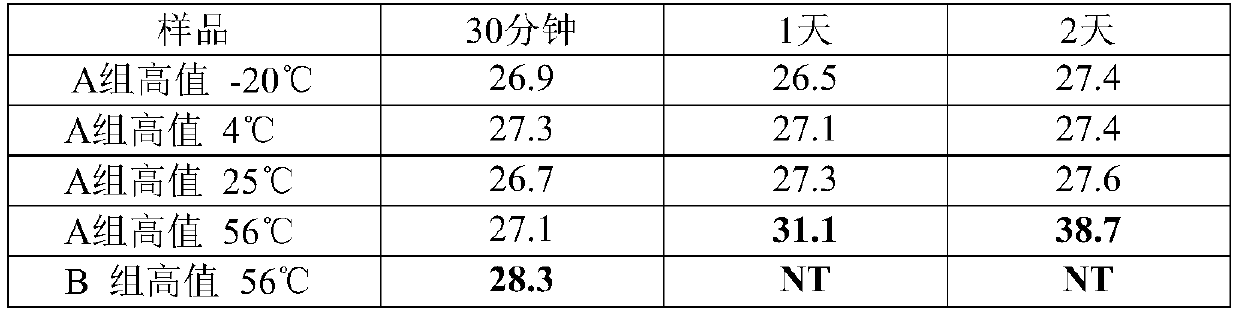
[0075]Using the recipe (S1) of the RNA virus inactivation preservation solution in Example 1 and the sample collection and processing methods in Example 1, the storage of the inactivation preservation solution in Example 1 at different temperatures was investigated. Among them, after air-drying the high-value samples on the surface of the swab, add 2 mL of the preservation solution (S1) of Example 1, vortex and shake for 10 minutes, take out the swab, and store the preservation solution at -20°C, 4°C, At 25°C and 56°C for a period of time, the control group was inactivated with the preservation solution of group B at 56°C (the control group is the method recommended by the Centers for Disease Control and Prevention (CDC) at this stage). 400 μL was taken out at each time point for extraction and RT-PCR test. From Table 3, we found that after treatment at 56°C for 30 minutes, the amount of templates detected in group A did not decrease, while the Ct of group B changed by more th...
Embodiment 3
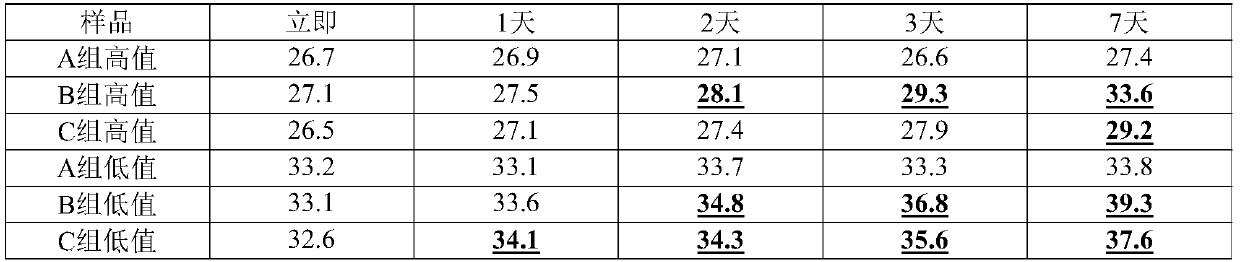
[0081] Adopt the formula (S1) of embodiment 1 RNA virus inactivation preservation liquid and the mode of embodiment 1 sample collection processing to three groups of preservation liquids: embodiment 1 formula preservation liquid S1 (group A), VTM aseptic virus transport liquid preservation liquid ( Group B) and BufferAVL (group C) were investigated with different kits, and the kits were respectively using MagMAX TM Viral RNA Isolation Kit (magnetic bead virus RNA extraction kit), provided by Qiagen Viral RNA Mini (column method virus RNA extraction kit) and Trizol method extraction kit (solvent method RNA extraction kit). The results are shown in Table 4. We found that whether Group A was used in high-value samples or low-value samples, after using different kits for extraction, the RT-PCR test results showed that the difference in Ct value was within 1; The solution is suitable for the column method, but the compatibility with the magnetic bead method is reduced, and it can...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com