A kind of carboxymethyl chitosan-rhein conjugate modified by TPGS and its synthesis process and application
A carboxymethyl chitosan and conjugate technology, which is applied to TPGS-modified carboxymethyl chitosan-rhein conjugate and its synthesis process and application fields, can solve the problems of poor absorption, drug resistance, The problem of low drug encapsulation rate, etc., achieves the effect of less toxic and side effects, promoting absorption, and inhibiting drug resistance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
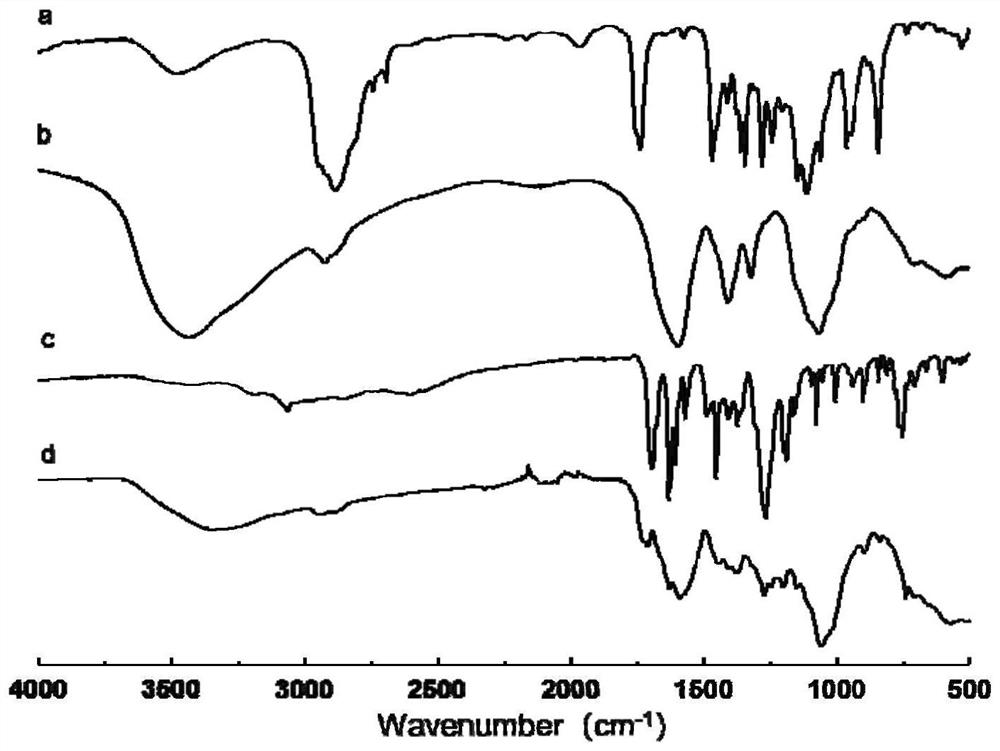
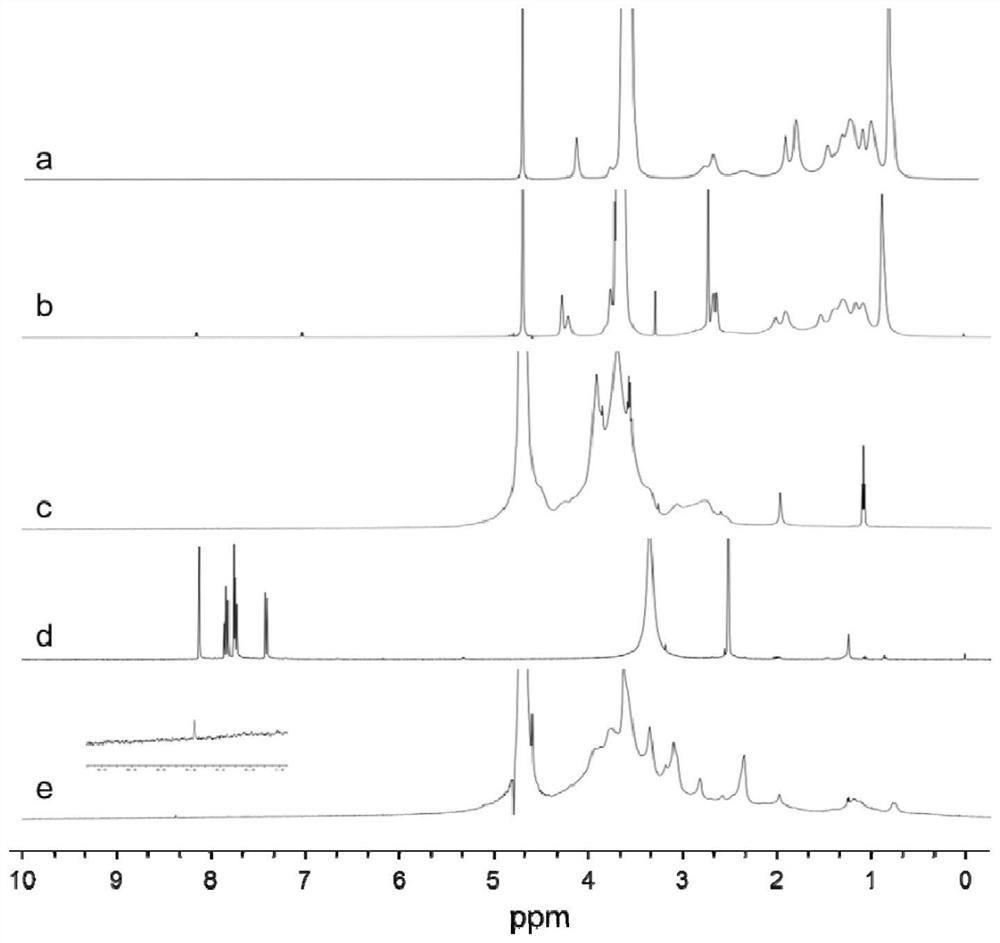
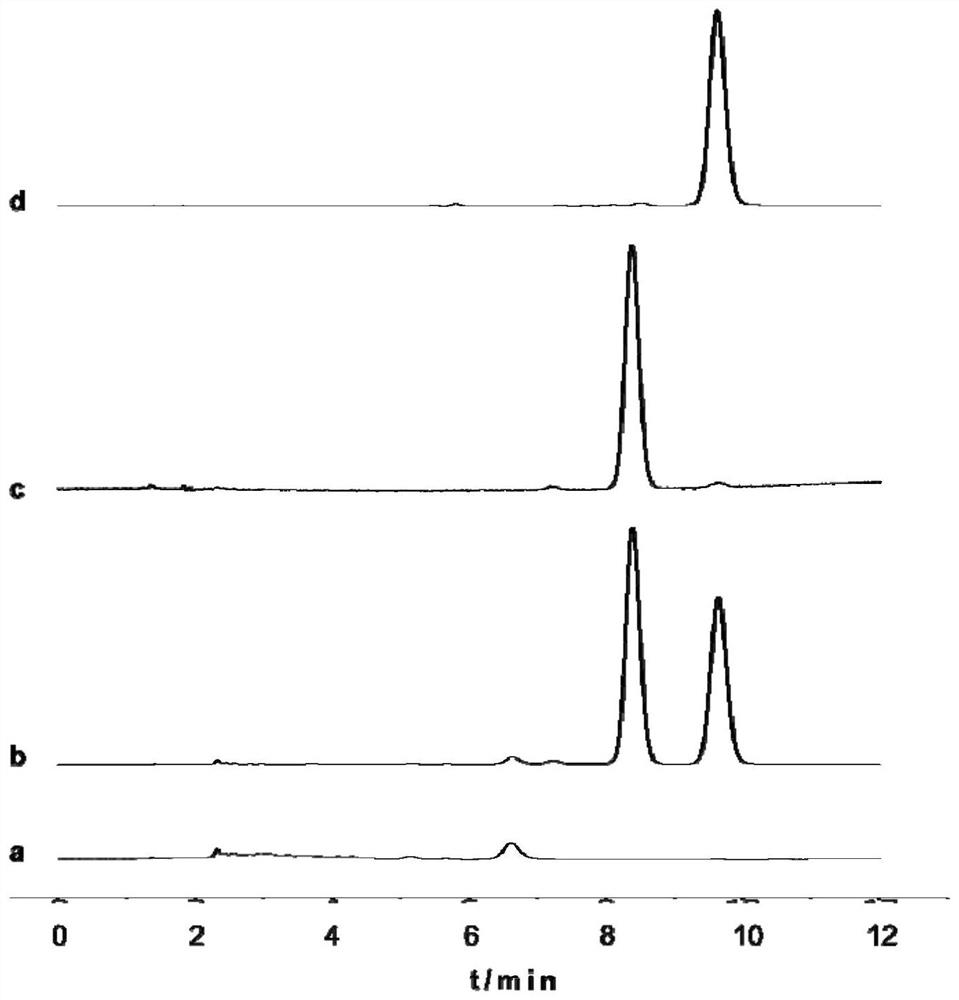
Image
Examples
Embodiment 1
[0055] The synthesis of embodiment 1TPGS-CR conjugate
[0056] 1. Synthesis of TPGS-SA intermediate
[0057] Weigh TPGS, succinic anhydride (SA) and 4-dimethylaminopyridine (DMAP), place in a vial, add triethylamine (TEA) dropwise, and the feeding amount is based on the molar ratio TPGS:SA:DMAP:TEA=1 :2:1:1, add appropriate amount of pyridine to dissolve. After magnetic stirring at room temperature for 24 h, pyridine was removed by rotary evaporation. The rotary evaporation residue was dissolved with an appropriate amount of dichloromethane, left to stand, centrifuged at 4000r / min for 10min, and the upper layer was evaporated to dryness to obtain a light yellow gel. The jelly was dissolved in an appropriate amount of water, stirred overnight by magnetic force, dialyzed for 72 hours, centrifuged to remove the precipitate, and the supernatant was freeze-dried to obtain a light yellow loose crude TPGS-SA.
[0058] 2. Synthesis of TPGS-CR conjugate
[0059] Weigh CMCS into a r...
PUM
| Property | Measurement | Unit |
|---|---|---|
| critical micelle concentration (mass) | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com