Branched-chain amino acids for the treatment of neuronal injury
An amino acid and composition technology, applied in the field of branched chain amino acid entities, can solve problems such as limited curative effect period
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0263] Example 1. Cytokinin levels in microglia treated with amino acid compositions.
[0264] Neuroinflammation and microglial activation are key mediators of repair and recovery after traumatic brain injury (TBI). However, recent clinical and laboratory data have demonstrated that TBI can cause persistent neuroinflammation and microglial activation, in some cases for many years, and can lead to chronic neurodegeneration, dementia, and encephalopathy. Prospective studies of TBI biomarkers in adults with severe TBI have demonstrated chronically increased serum levels of IL-1β, IL-6, CXCL8, IL-10, and tumor necrosis factor (TNFα).
[0265] Pups were decapitated, brains were removed from the skulls, cortices were excised and collected in calcium- and magnesium-free Hanks' balanced salt solution (CMF-HBSS) on ice. Mince cortex with a sterile razor blade in mincing solution (CMF-HBSS) and 2 ml of digestion solution (0.25% trypsin in CMF-HBSS) in a 15 ml sterile plastic tube in a ...
Embodiment 2
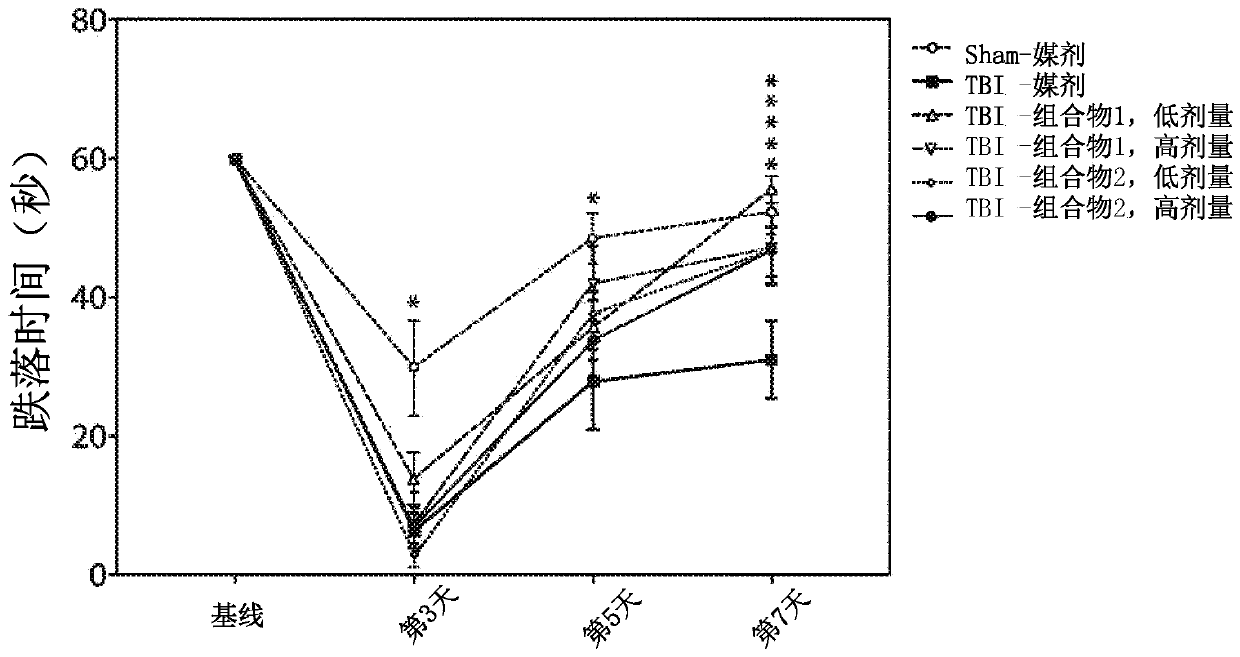
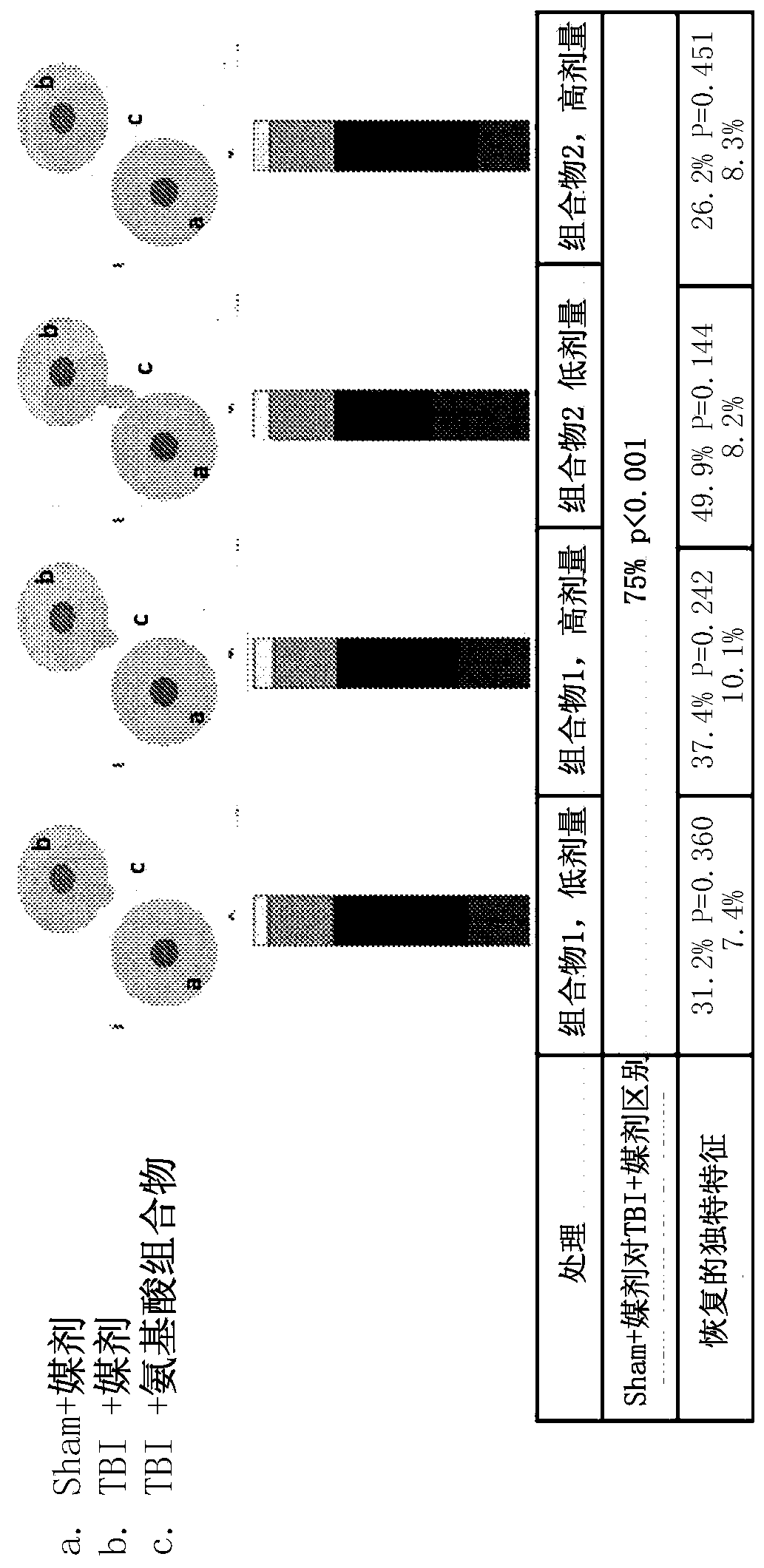
[0278] Example 2. Efficacy assessment of amino acid compositions in a rat model of TBI.
[0279] Adult male Sprague-Dawley rats (250-350 g) from Charles River were used. Animals were housed 2 per cage in polycarbonate rat cages equipped with microisolators and acclimated for up to 7 days. All rats were inspected, handled and weighed to assess adequate health and fitness before starting the study. A 12 / 12 light / dark cycle was maintained during the study. The room temperature was maintained between 20°C and 23°C and the relative humidity was maintained at about 50%. Feed and water were provided ad libitum for the duration of the study. The animals are randomly assigned to treatment groups.
[0280] Among several animal models of TBI, the controlled cortical impingement (CCI) model is the most commonly used and the only one commercially available. Compared with other models, the CCI model can control all mechanical factors such as the time, speed and depth of impact, and in ...
Embodiment 3
[0295] Example 3. Treatment of Individuals Suffering from TBI with Amino Acid Compositions.
[0296] A feature of the studies described herein was the administration of compositions comprising amino acids to patients suffering from TBI. Adult individuals 18 to 35 years old (inclusive) suffering from mild traumatic brain injury resulting from any sports-related event or blunt trauma to the head will be administered the amino acid composition. The composition contained L-leucine, L-isoleucine, L-valine, N-acetylcysteine (NAC) and acetyl-L-carnitine (ALCAR) (Table 8). The individual will receive approximately 17.1 g of the amino acid composition twice daily for a total of 34.2 g per day.
[0297] Table 8. Exemplary components and amounts of amino acid compositions for treating individuals suffering from TBI.
[0298]
[0299] This study was primarily used to evaluate the safety, tolerability and pharmacokinetics of the composition administered for 8 days in adult individua...
PUM
| Property | Measurement | Unit |
|---|---|---|
| depth | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com