Monoclonal antibody for detecting novel coronavirus and application of preparation kit
A monoclonal antibody, coronavirus technology, applied in biological testing, antiviral immunoglobulin, applications, etc., can solve the problems of lack of antibody duration, systematic research, etc., to achieve high specificity, broad market prospects, titer high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] The epitope optimization of embodiment 1N protein
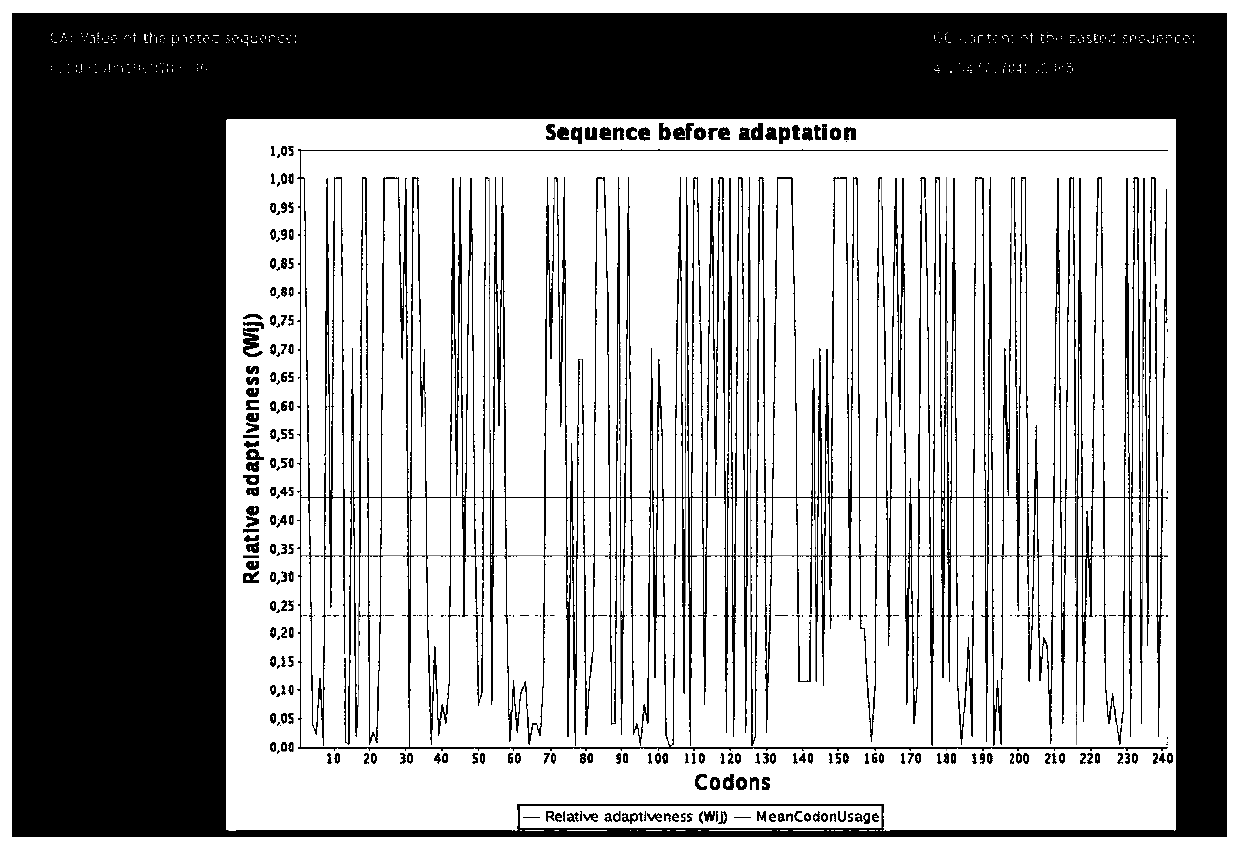
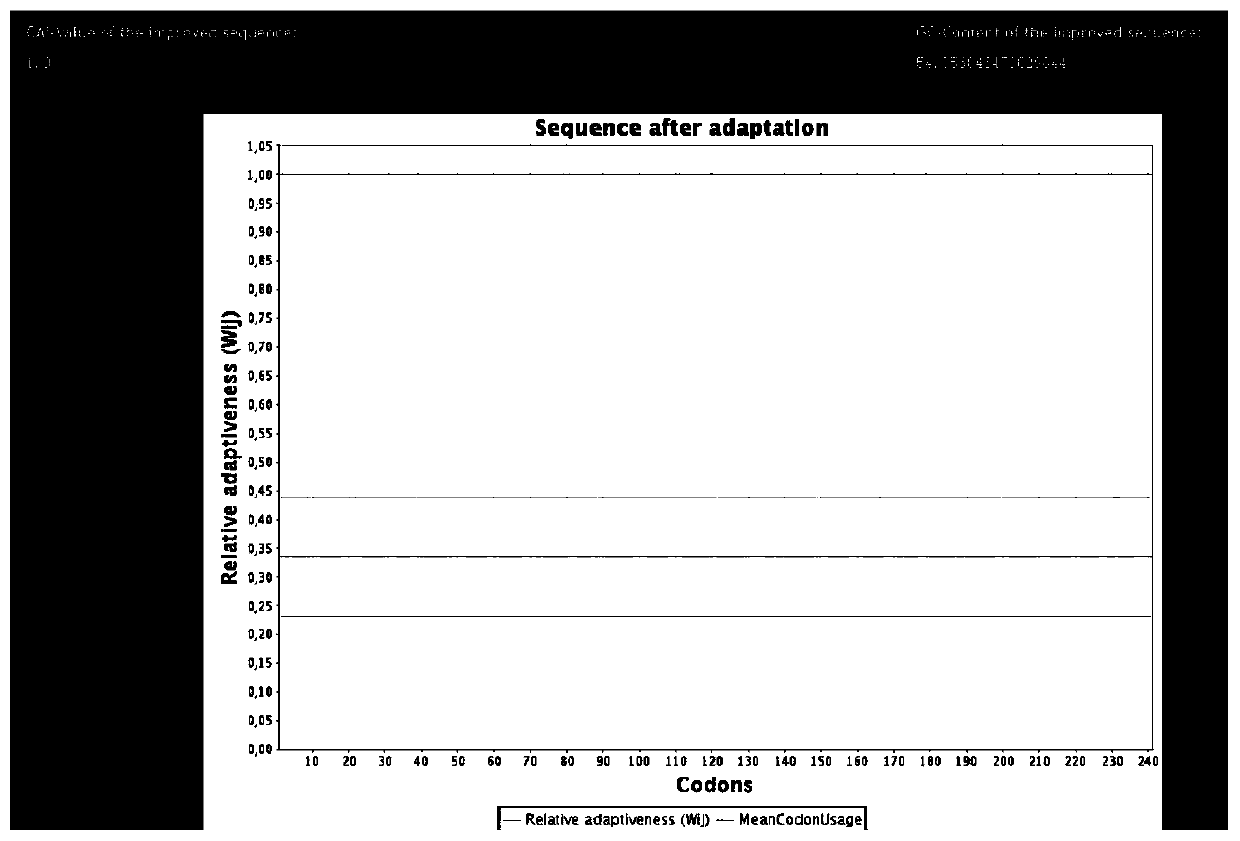
[0028] According to the published whole genome sequence of 2019-nCoV, an optimized N protein epitope amino acid sequence was designed for the N protein amino acid sequence peptide. Through Clustal W (Version1.81) software multiple sequence comparison of 5 amino acid sequences of different strains such as Chinese strain and Italian strain, the sequence was analyzed, and the immunogenicity, hydrophilicity, surface possibility and related secondary structure were analyzed according to DNAstar . Through Modeller homology modeling, the three-dimensional structure of the protein was constructed, and the surface possibility of the immunogenic polypeptide was further analyzed. The antibody-binding epitope peptide was simulated by the MOE software, and the interacting amino acids were analyzed, and the N protein sequence with good immunogenicity was screened. , and from Figure 1A and Figure 1B In comparison, Figure 1B Th...
Embodiment 2
[0034] Expression of embodiment 2N protein
[0035] Design specific primers according to the optimized N protein nucleotide sequence, respectively add protective bases and restriction endonucleases at the 5' end of the primers, and the primer sequences are as follows:
[0036] F:5'-CGC GGATCC ATGAAAGACCTGTCTCCGCG-3' (SEQ. ID. NO. 3):
[0037] R:5'-CCG GAATTC GTCGTCCAGTTTGATAGCAC-3' (SEQ.ID.NO.4):
[0038] Using the optimized N protein gene synthesized by the whole gene as a template (Shanghai Sangong Synthetic), PCR amplification was carried out with the above-mentioned specific primers.
[0039] The PCR reaction program was: pre-denaturation at 94°C for 2 min; denaturation at 94°C for 10 sec, annealing at 55°C for 35 sec, extension at 67°C for 1 min and 10 s, 35 cycles; extension at 68°C for 7 min, and storage at 4°C.
[0040] PCR reaction system: 2×PCR Buffer 12.5μL, 2mM dNTPs 5μL, 10mM R Primer 0.75μL, 10mM F Primer 0.75μL, N DNA 1μL, dHO 2 O 5 μL.
[0041] The ampl...
Embodiment 3
[0044] The preparation of embodiment 3 monoclonal antibody
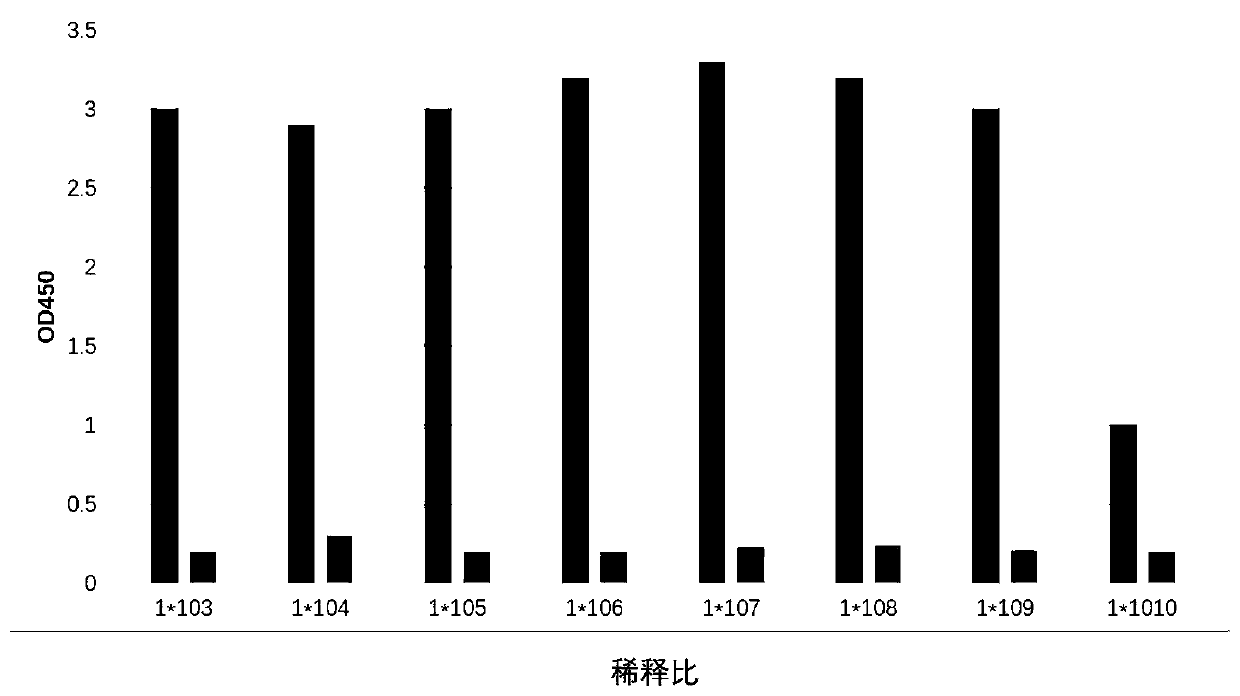
[0045] Fifteen Balb-c mice, female, 8 weeks old, with a body mass of (20±2) g, were provided by the Experimental Animal Center of Yangzhou University. Randomly divided into 3 groups, 5 in each group. Take the N protein obtained in Example 2 as the immunogen, fully emulsify it with an equal amount of complete Freund's adjuvant, inject the antigen at multiple points subcutaneously in mice at an amount of 70 μg / mouse, and emulsify the antigen with incomplete Freund's adjuvant 2 weeks later The second immunization was carried out, and after the third immunization, the ELISA titer was detected to be >1:10,000, and 50 g of antigen was used for intraperitoneal impact immunization, and fusion was performed 3 to 4 days later.
[0046] Aseptically take the spleen of the mouse, prepare splenocytes, and fuse the splenocytes with myeloma SP2 / O cells in the logarithmic growth phase with a volume fraction of 50% PEG4000; the fused...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com