Transeliminase encoding gene and enzyme and preparation and application
A technology of pectate lyase and gene, applied in the direction of lyase, carbon-oxygen lyase, application, etc., can solve the problems of weak degradation activity of pectin polysaccharides and unsuitable for large-scale application, and achieve good activity and efficient degradation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1 Pectate lyase Ap Pel full-length gene cloning
[0044] The total RNA of Aspergillus parasitica was extracted according to the operating procedure of the Column Fungal Total RNA Extraction and Purification Kit (Shanghai Sangong), and the first-strand cDNA was synthesized according to the operating procedure of the RevertAid Frist Strand cDNA Synthesis Kit (Thermo Scientific). After performing multiple sequence alignment analysis on the pectate lyase gene sequence in The National Center for Biotechnology Information (NCBI) database, degenerate primers were designed:
[0045] Ap Pel-F: 5'-CAGTCTCGAGAAAAAGACAGAAAGTCTCTGGTGCC-3';
[0046] Ap Pel-R: 5'-ATCTTCTAGAGATCTTGCCCGCACCAGCATTC-3',
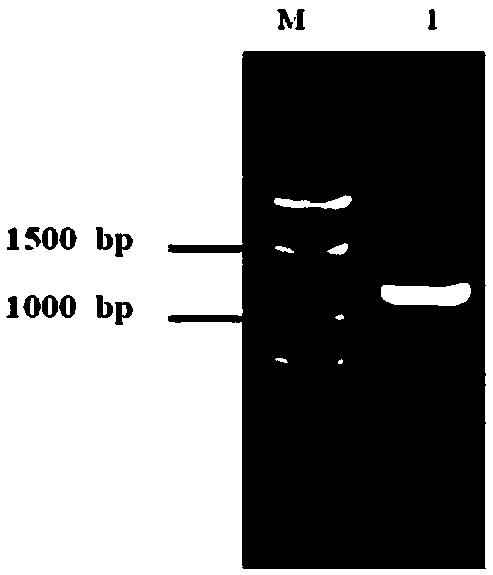
[0047] Using the first-strand cDNA of Aspergillus parasitica as a template, the gene sequence encoding pectate lyase protein (excluding the signal peptide gene) was amplified. The PCR reaction conditions were: 98°C for 3min, 1 cycle; 98°C for 10s, 55°C for 15s, 72°C for 2min, 3...
Embodiment 2
[0048] Example 2 Analysis of pectate lyase gene sequence
[0049] The sequencing results were analyzed using Basic Local Alignment Search Tool (BLAST) in the GenBank database, DNAMAN software was used for multiple sequence alignment, and Vector NTI was used to analyze sequence information.
[0050] The coding region of the obtained pectate lyase gene (named Ap Pel) is 1086 bp long, and its nucleotide sequence is shown in SEQ ID NO 1. Ap Pel encodes 361 amino acids and a stop codon, its amino acid sequence is shown in SEQ ID NO 2, the theoretical molecular weight of the protein is 38.10kDa, and the predicted isoelectric point is 8.90. The amino acid encoded by ApPel has a pectate lyase central domain, which indicates that ApPel is a member of the polysaccharide lyase family.
Embodiment 3
[0051] Example 3 Heterologous expression and purification of pectate lyase Ap Pel in Pichia pastoris
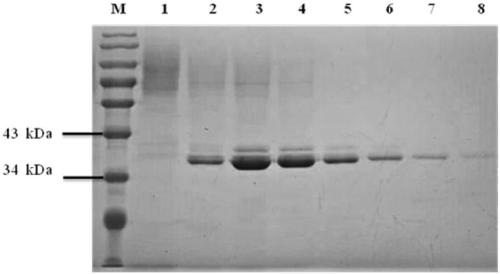
[0052] Pectate lyase Ap Pel was induced and expressed according to the method of Pichia pastoris expression manual. The expression and purification of pectate lyase Ap Pel were detected by polyacrylamide gel electrophoresis, and the results were as follows: figure 2 As shown, the purified pectate lyase Ap Pel presents a single band on the electrophoresis gel, and its position (about 38 kDa) coincides with the predicted molecular weight (38.10 kDa).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Theoretical molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com