Application of human symbiotic florae in improving response of tumors to immunotherapy
A technology of intestinal flora and liquid, applied in the field of biomedicine, can solve the problems of delaying the treatment time of patients, unaffordable for ordinary patients, poor treatment effect of patients, etc., and achieve the goal of improving response, enhancing tumor suppression effect, and low toxic and side effects Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0086] An object of the present disclosure is to provide a method for preparing a whole bacterial composition of healthy human intestinal flora, characterized in that the method comprises the following steps:
[0087] Add healthy human feces to sterile deoxygenated saline at a mass volume ratio of 1:3-5, and stir the sample;
[0088] Homogenize the sample;
[0089] Filter the homogenized liquid step by step;
[0090] The filtrate was centrifuged twice and resuspended, and the resulting suspension was the whole bacterial composition of the human intestinal flora;
[0091] The method is carried out under anaerobic conditions.
[0092] In a preferred embodiment, the first centrifugal condition is 0-25°C, 50g-200g, centrifuged for 1-15 minutes; the second centrifuged condition is 0-25°C, 4000g-5500g, centrifuged for 1-15 minutes minute;
[0093] The temperature of the first and second centrifugation is preferably 0-15°C, or 1-10°C, or 2-9°C, or 3-8°C, or 4-7°C, or 5-6°C, most ...
Embodiment 1
[0122] Example 1: Preparation of whole bacterial composition of healthy human intestinal fecal flora
[0123] Choose healthy people aged 32 and pass health screening and clinical examination: blood tests for HIV, hepatitis B, EB virus (epstein-barr virus, EBV) and other infections; the body has no digestive tract diseases and other diseases that will affect intestinal flora such as metabolism Syndrome, etc.; no rotavirus, norovirus, Salmonella, Shigella, carbapenem-resistant Enterobacteriaceae, methicillin-resistant Staphylococcus aureus, vancomycin-resistant Enterococcus, Extended-spectrum beta-lactamase-producing Enterobacteriaceae, Candida albicans, Cryptosporidium and Cyclosporidium infection; regular meals, regular work and rest.
[0124] Use a specific sterile stool collection box to collect fresh stool samples, cover the lid after sampling, send it to the laboratory for weighing, and record the quality; put the sampling box in the transfer window of the anaerobic operat...
Embodiment 2
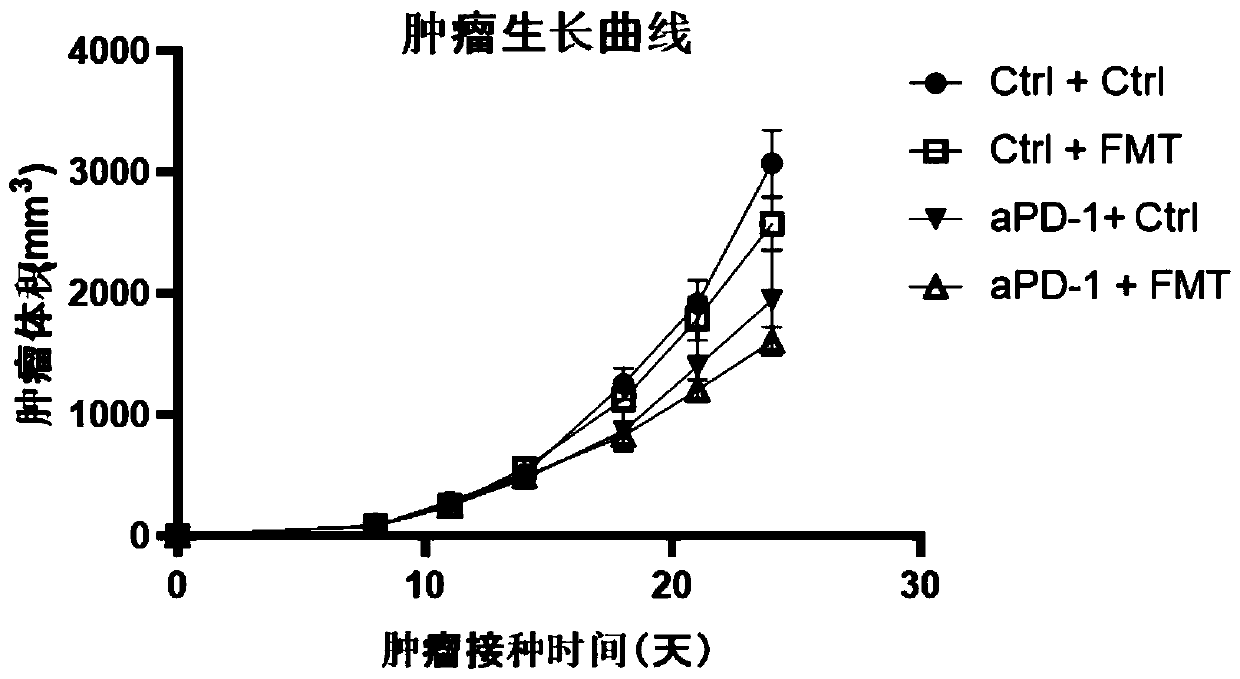
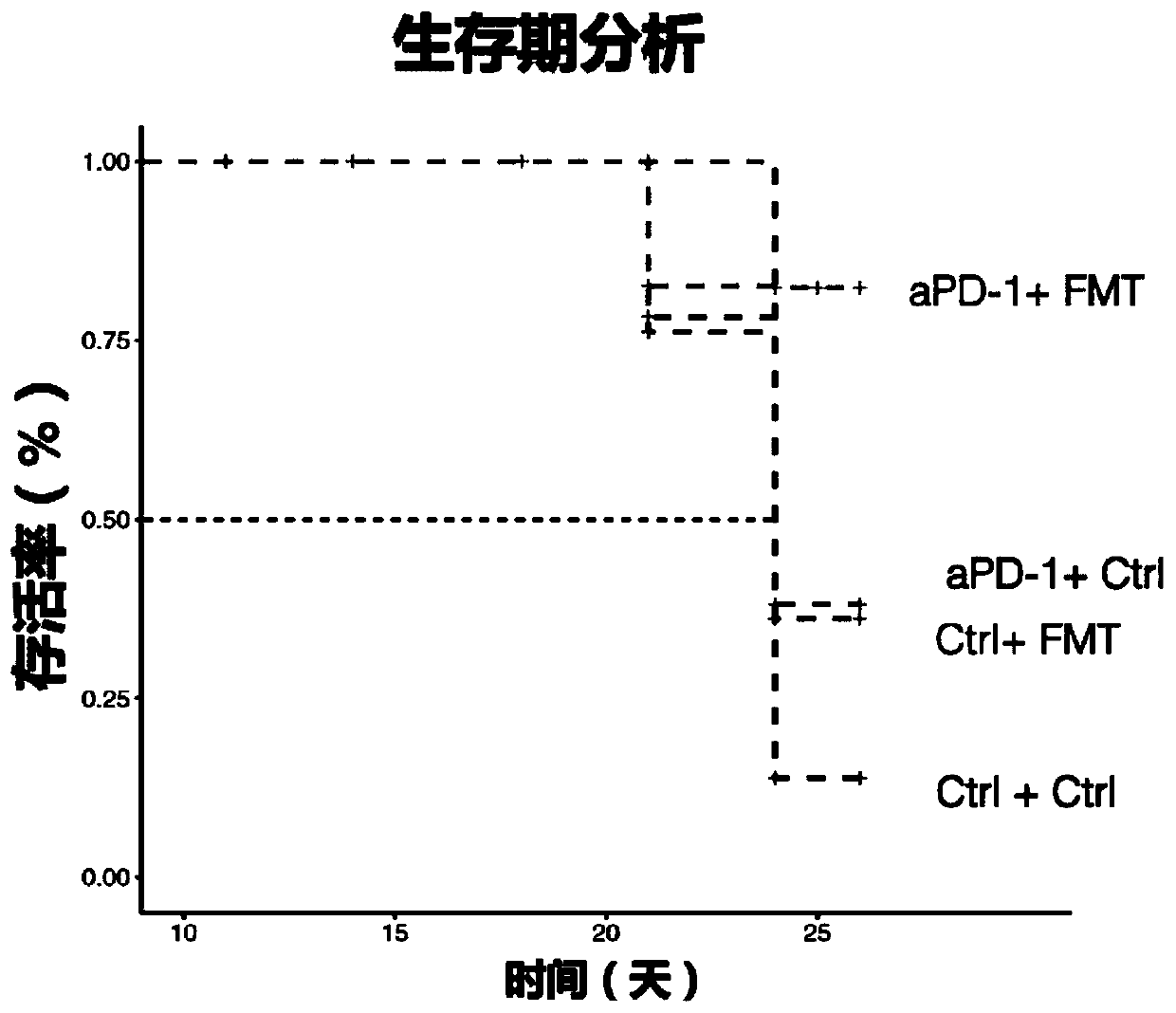
[0129]Example 2: Whole bacteria in the intestinal flora of healthy people combined with PD-1 immunosuppressant for the treatment of gastrointestinal tumors
[0130] Test drug Rat IgG2a
[0131] Supplier: Sino-US Crown Biotechnology Co., Ltd.
[0132] Item No.: CVP039
[0133] Batch number: 0119L220
[0134] Packing: 2.8mg / ml, 96mg
[0135] Storage temperature:
[0136] Test drug aPD-1 antibody (RMP1-14)
[0137] Supplier: Sino-US Crown Biotechnology Co., Ltd.
[0138] Item No.: CVP033
[0139] Batch number: 0119L225
[0140] Packing: 4.1mg / ml, 96mg
[0141] Storage temperature:
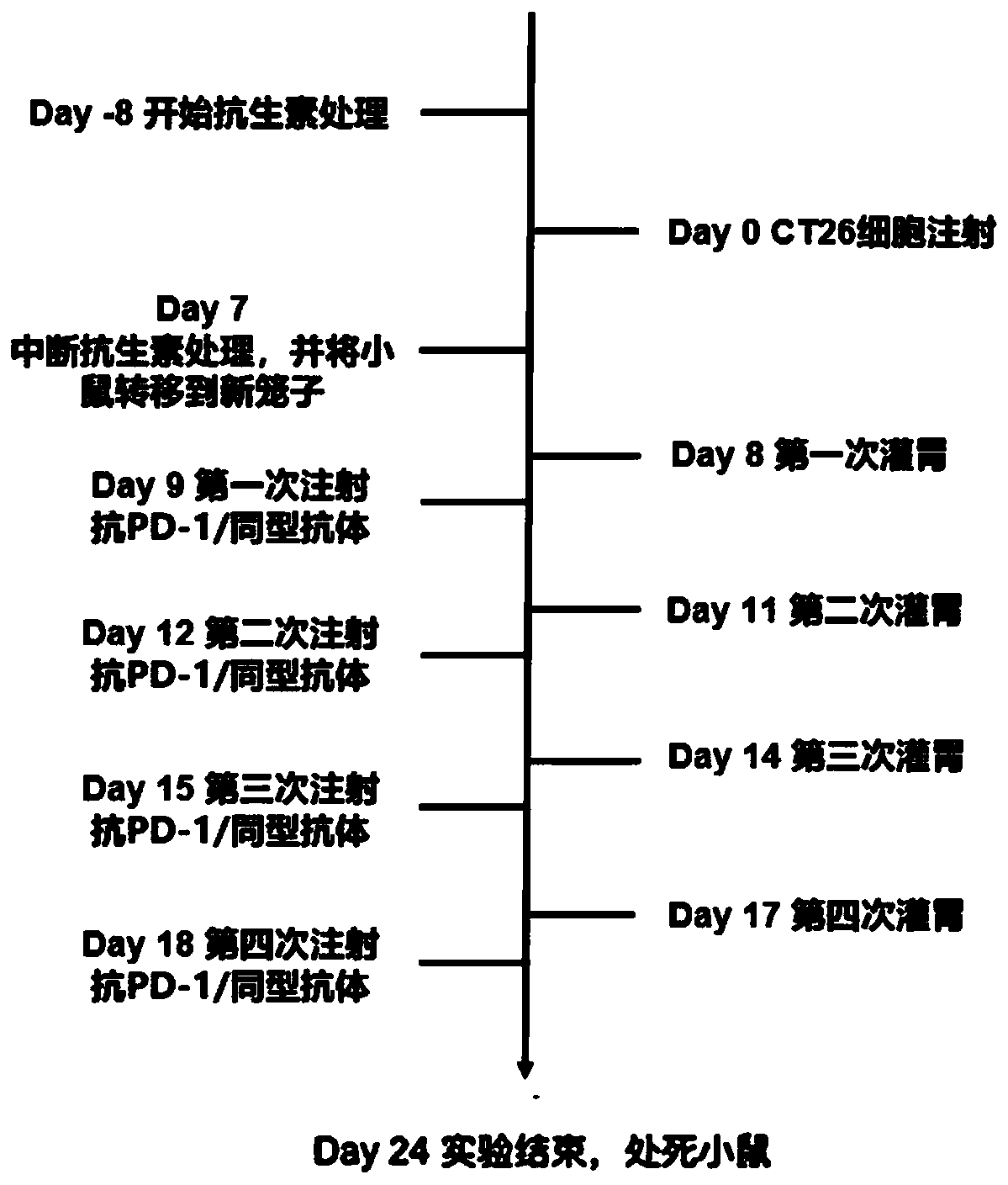
[0142] antibiotic
[0143] Day-8 to Day-4: The formula includes Ampicillin 1mg / ml, Neomycin 1mg / ml, Metronidazole 1mg / ml and Vancomycin 0.5mg / ml . Antibiotics were added in proportion to sterile water for mice to drink, and the antibiotic treatment time was 4 days, and the water bottles containing antibiotics were changed every three days.
[0144] Day-3 to Day 7: The formula includes...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mean tumor volume | aaaaa | aaaaa |
| Mean tumor volume | aaaaa | aaaaa |
| Mean tumor volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com