mRNA and vaccine for coding a SARS-CoV-2 viral antigen and preparation method of vaccine
A virus antigen, sars-cov-2 technology, applied in the field of vaccines, can solve the problems of complex preparation process, virus production, hidden safety hazards, etc., and achieve the effects of simple preparation process, easy industrialization, and high safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0059] The preparation method of the vaccine that uses cationic liposome as delivery formulation to deliver mRNA preferably includes: mixing mRNA and cationic liposome in a working solution to obtain the vaccine. Wherein the working liquid preferably includes deionized water, ultrapure water, sodium chloride solution or PBS. The concentration of mRNA in the working solution is preferably 0.01-0.5 mg / ml. The working solution is preferably PBS, and when the working solution is PBS, the mass ratio of cationic liposomes to mRNA is 1:1-4:1.
[0060] In some preferred embodiments, the preparation method of cationic liposomes includes providing a solution containing neutral helper lipids and cationic lipids, removing the solvent, drying, hydrating and sonicating the solution in sequence to obtain cationic liposomes . Wherein the solvent preferably includes a mixture of chloroform and / or dichloromethane and methanol; the drying time is preferably 1.5 to 2.5 hours; the water splash p...
Embodiment 1
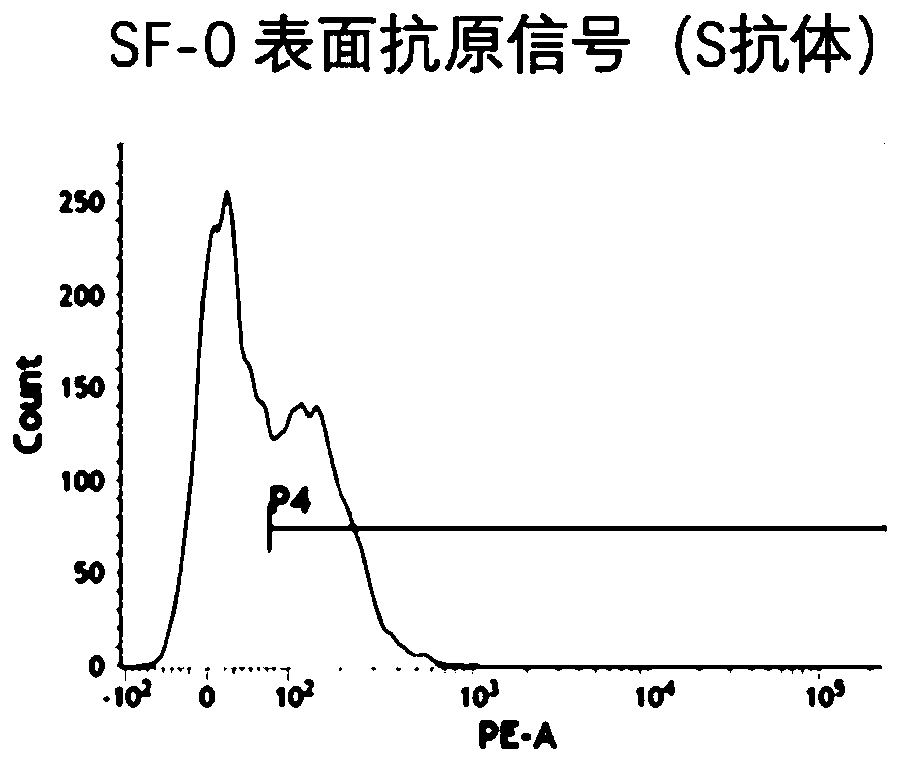
[0063] According to the natural coding region sequence of the SARS-CoV-2 virus S protein, this embodiment designs the full-length sequence of the SARS-CoV-2 virus S protein (SF mRNA sequence). In addition to the coding region, the SF mRNA sequence features also include DNAH2 5 'UTR, HBA23' UTR and 50 polyA, SF-1 and SF-2 mRNA sequences were optimized in the S protein coding region, compared with SF-0 mRNA, the GC content was increased, but kept consistent in the UTR region, the sequence information is as follows Table 1 shows.
[0064] Table 1
[0065]
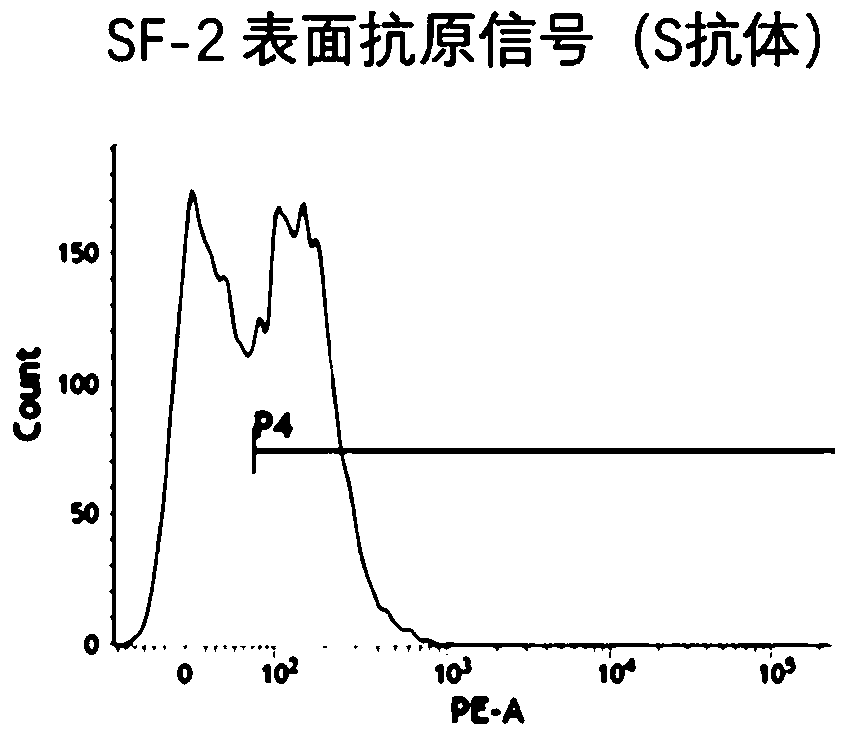
[0066] The target antigen production detection method (flow cytometry) is as follows:
[0067] 1. In order to detect the expression of SARS-CoV-2 virus S protein mRNA in human cells and the location on the cell membrane, in this embodiment, 293 cells cultured for more than 24 hours were digested and planted in a 6-well plate, and the cell density was controlled at 400,000 per hole.
[0068] 2. After incubating the six-we...
Embodiment 2
[0078] The mRNA encoding the full length of the S protein (SF mRNA is shown in the sequence shown in SEQ ID NO.13), the mRNA encoding the S1 subunit (the sequence shown in SEQ ID NO.14) and the receptor binding region RBD encoding the S protein mRNA (sequence shown in SEQ ID NO.15) transfected cells, detection of S, S1 and RBD protein expression in cells, the results are as follows Figure 4 As indicated, HEK293 cells transfected with each mRNA 24 hours later were lysed, loaded on SDS-PAGE gel with 10 μg of total protein, immunoblotted with anti-SARS-S1 protein antibody, and labeled with goat anti-rabbit-HRP secondary antibody , and then color. Protein expression was quantified using the internal reference b-actin. Cells not transfected with mRNA served as a negative control. The expression of full-length S protein, S1 subunit and RBD protein could be detected.
[0079] Using the target antigen production detection method in Example 1 to detect the expression of the antigen...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com