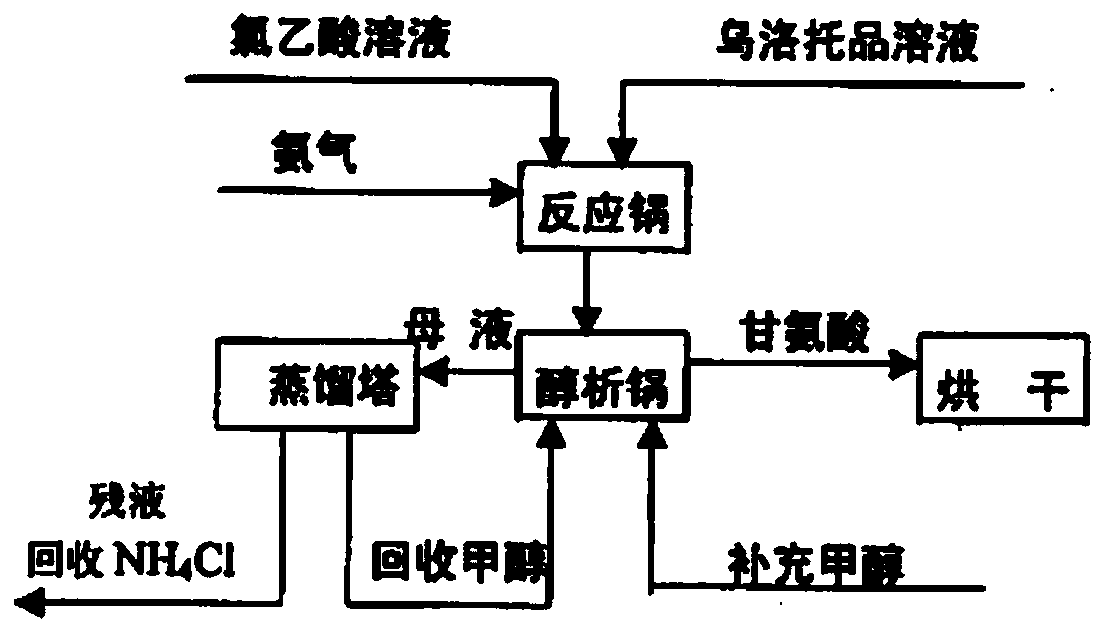
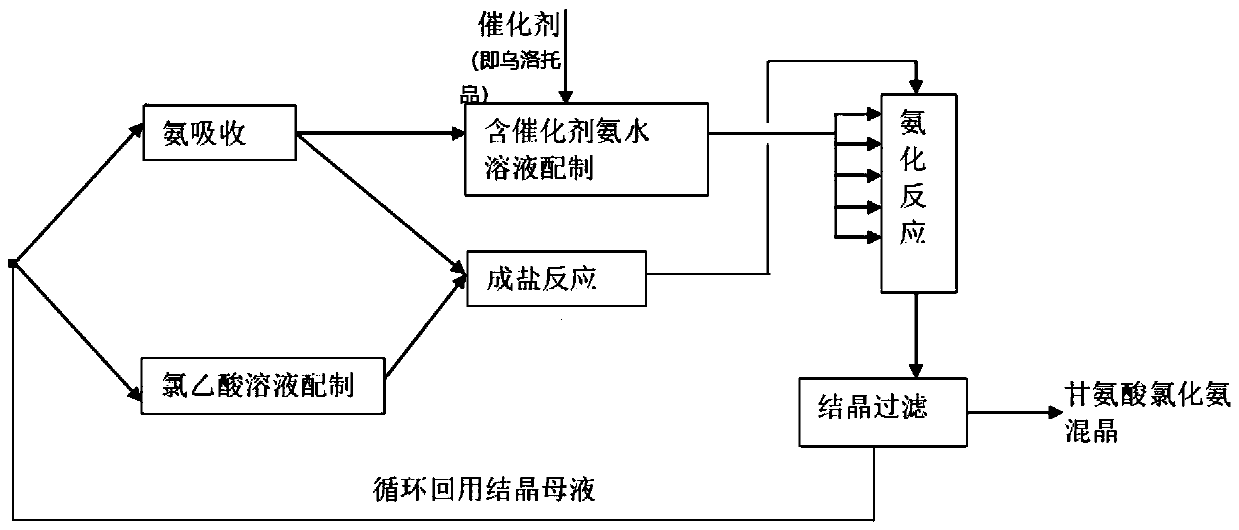
Continuous glycine synthesis method
A synthesis method, glycine technology, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve problems such as quality not up to product standards, high consumption, difficult ammonium chloride treatment, etc., and achieve environmental protection in production Problems, the effect of unit consumption reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Such as Figure 2-3 Shown is the production process diagram and product diagram of glycine of the present invention. (1) Dissolve chloroacetic acid in the mixed crystal mother liquor (in the beginning of production, replace the mixed crystal mother liquor with water) to make a chloroacetic acid solution with a mass concentration of 50%.
[0037] (2) Dissolve liquid ammonia in mixed crystal mother liquor to prepare ammonia water with a mass concentration of 15%, and the temperature of the ammonia water is not higher than 30°C.
[0038] (3) Chloroacetic acid solution and a part of ammonia water undergo a salt-forming reaction in the salt-forming reactor. In the salt-forming reaction, the molar ratio of chloroacetic acid to ammonia is 1.0 to obtain solution A; the reaction temperature is controlled at 30°C, and the reaction pH is controlled at 5.0.
[0039] (4) Dissolve a certain amount of urotropine solution in the remaining ammonia water, so that the total mass concentr...
Embodiment 2
[0043] (1) Dissolve chloroacetic acid in the mixed crystal mother liquor (in the beginning of production, replace the mixed crystal mother liquor with water) to make a chloroacetic acid solution with a mass concentration of 65%.
[0044](2) Dissolve liquid ammonia in the mixed crystal mother liquor to prepare ammonia water with a mass concentration of 25%; the temperature of the ammonia water is not higher than that of 30°C.
[0045] (3) Chloroacetic acid solution and a part of ammonia water undergo a salt-forming reaction in the salt-forming reactor. In the salt-forming reaction, the molar ratio of chloroacetic acid to ammonia is 2 to obtain solution A. The reaction temperature is controlled at 45°C and the reaction pH is controlled at 7.0.
[0046] (4) Dissolve a certain amount of urotropine solution in the remaining ammonia water, so that the total mass concentration of urotropine is 23%; the molar ratio of urotropine to ammonia is 0.3.
[0047] (5) The ammonia water contai...
Embodiment 3
[0050] (1) Dissolve chloroacetic acid in the mixed crystal mother liquor (in the beginning of production, replace the mixed crystal mother liquor with water) to make a chloroacetic acid solution with a mass concentration of 55%.
[0051] (2) Dissolve liquid ammonia in mixed crystal mother liquor to prepare ammonia water with a mass concentration of 20%, and the temperature of the ammonia water is not higher than 30°C.
[0052] (3) Chloroacetic acid solution and a part of ammonia water undergo a salt-forming reaction in the salt-forming reactor. In the salt-forming reaction, the molar ratio of chloroacetic acid to ammonia is 0.8 to obtain solution A. The reaction temperature is controlled at 40°C and the reaction pH is controlled at 6.0.
[0053] (4) Dissolve a certain amount of urotropine solution in the remaining ammonia water, so that the total mass concentration of urotropine is 20%; the molar ratio of urotropine to ammonia is 0.1.
[0054] (5) The ammonia water containing ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com