TA polypeptide, TA polypeptide-modified drug delivery system, preparation method of TA polypeptide-modified drug delivery system and application of TA polypeptide and TA polypeptide-modified drug delivery system
A peptide modification and delivery system technology, applied in the field of medicine, can solve problems such as easy inactivation, inconvenience to patients, unstable physical and chemical properties, etc., and achieve strong target binding ability and the effect of inhibiting proliferation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0101] Example 1 Synthesis and characterization of TA, Fluorescein-TA.
[0102] (1) Put a certain amount of resin into the reactor, add dichloromethane (DCM) to swell for half an hour, then remove the DCM, add the first amino acid in the sequence and diisopropylethylamine (DIEA), an appropriate amount DMF, nitrogen bubbling reaction for 60min. Then add methanol, react for half an hour, remove the reaction solution, and wash with DMF and MEOH;
[0103] (2) Add the second amino acid in the sequence, 1-hydroxyl, benzo, trichlorazole tetramethylhexafluorophosphate (HBTU) and DIEA to the reactor, react with nitrogen bubbles for half an hour, wash off the liquid, indene Triketone detection followed by capping with pyridine and acetic anhydride. Finally, wash, add an appropriate amount of decapping solution to remove the 9-fluorenylmethoxycarbonyl (Fmoc) protecting group, wash, and detect ninhydrin; add different amino acids in the sequence according to the above method until th...
Embodiment 2
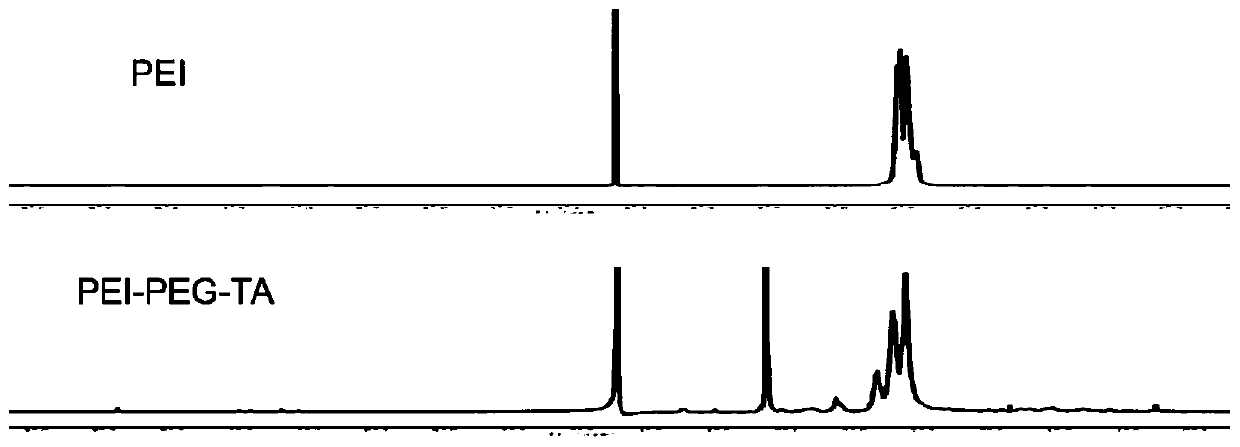
[0109] Example 2 Synthesis and characterization of PEI-PEG-TA.
[0110] Weigh 13.50 mg of TA and 9.0 mg of NHS-PEG-MAL and dissolve them in 500 μL DMSO, react for 12 h under nitrogen protection, transfer the reaction product to a Sephadex S30 gel column, and buffer it with phosphate buffer at a flow rate of 1 ml / min Liquid flow relative to the product was separated and purified, and the product NHS-PEG-TA was lyophilized. Weigh 15mgPEI and dissolve in 0.1mM pH
[0111] In the phosphate buffer solution of 7.2, weigh 6.40mg NHS-PEG-TA and dissolve it in 500μL DMSO, add it dropwise to the PEI solution under the condition of stirring, and react overnight under the protection of nitrogen, with a molecular weight cut-off of 8000-14000Da The dialysis bag was dialyzed for 48h, and the product was obtained by freeze-drying. 1 H NMR characterizes the final product as image 3 shown.
Embodiment 3
[0112] Example 3 The binding ability of TA to VEGFR-2 and NRP-1 receptor protein was determined.
[0113] (1) Using surface plasmon resonance (SPR) technology, ① coupled protein. The best coupling solutions for VEGFR-2 and NRP-1 proteins were selected by pH scouting test: sodium acetate solution with pH 4.0. First use EDC / EHS to activate the carboxyl group of the CM5 chip, dilute the VEGFR-2 and NRP-1 proteins to 50 μg / mL with sodium acetate solution at pH 4.0, and form a covalent bond by reacting the amino group of the protein with the carboxyl group of the CM5 chip Coupled to channels 2 and 4 in the same way, and then blocked with ethanolamine; channels 1 and 3 were only activated by EDC / NHS and blocked with ethanolamine as reference channels. ②Binding force analysis. Through the surface test, the analyte TA mother solution was diluted to 0.25 μmoL as the highest concentration of injection, and six concentrations were serially diluted, injected at a flow rate of 30 μL / m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com