Kit for quantitatively detecting content of pregnancy-related protein A
A technology for quantitative detection of pregnancy-related protein, applied in the field of kits for quantitative detection of pregnancy-related protein A content, can solve the problems of many influencing factors, many detection steps of ELISA method, and long time-consuming, so as to achieve reliable clinical reference value and reduce non-specific Heterotropic adsorption, good linear relationship effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
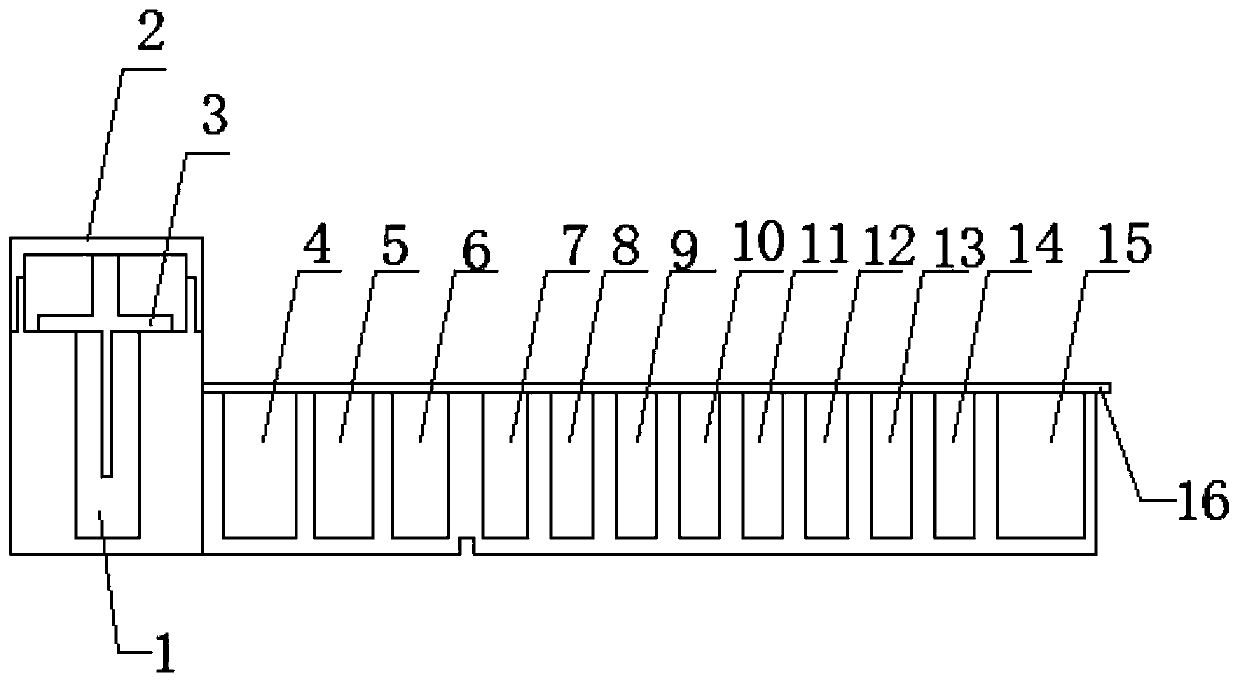
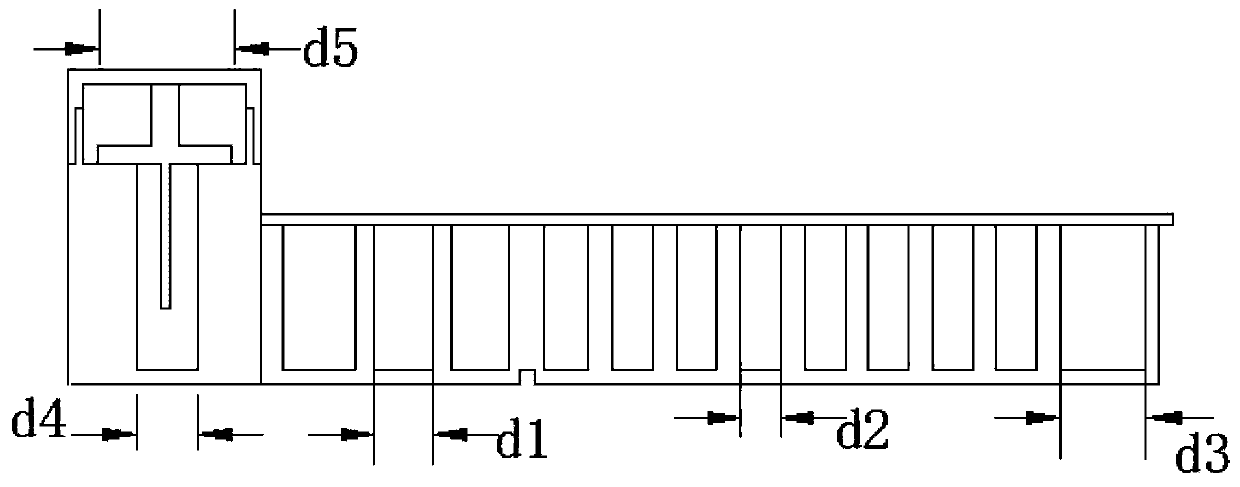
[0025] A kit for quantitatively detecting the content of pregnancy-associated protein A, comprising a kit main body, a sealing cover, a protective cover, and a reagent. The kit main body is sequentially provided with solid-phase carrier holes, sample holes, detection antibody holes, fluorescein holes, washing Hole, reading hole, the inside of the solid phase carrier hole is a T-shaped structure, a protective cover is provided above the solid phase carrier hole, the protective cover and the solid phase carrier hole are connected by threads, and an internal thread is provided on the inner side of the lower end of the protective cover. The upper end of the carrier hole is provided with an external thread, the external thread matches the internal thread, a solid phase carrier is provided in the solid phase carrier hole, a serum sample is installed in the sample hole, and a biotin-labeled detection antibody is installed in the detection antibody hole , the fluorescein-labeled strept...
Embodiment 2
[0033] A kit for quantitatively detecting the content of pregnancy-associated protein A, comprising the steps of:
[0034] (1) Solid phase carrier coated with capture antibody: soak the lower end of the quartz needle in the polysaccharide complex for 1-3 minutes, freeze-dry at 4°C, and dilute the pregnancy-associated protein A capture antibody to 1mg with 0.01M PBS buffer / mL, place the lower end of the dried quartz needle in the pregnancy-associated protein A capture antibody solution, react for 2 hours at 24°C for labeling, wash 3 times with 0.1M PBS buffer after labeling, and then place in blocking solution Blocking for 8-12 hours, the blocking solution is Tris-HCL buffer containing 1% BSA, 0.1% casein, and 3% sucrose, and the solid phase carrier coated with the capture antibody is prepared;
[0035](2) Biotin-labeled detection antibody: Dilute the pregnancy-associated protein A detection antibody to 1mg / mL with 0.01M PBS buffer, and add biotin according to the mass ratio a...
Embodiment 3
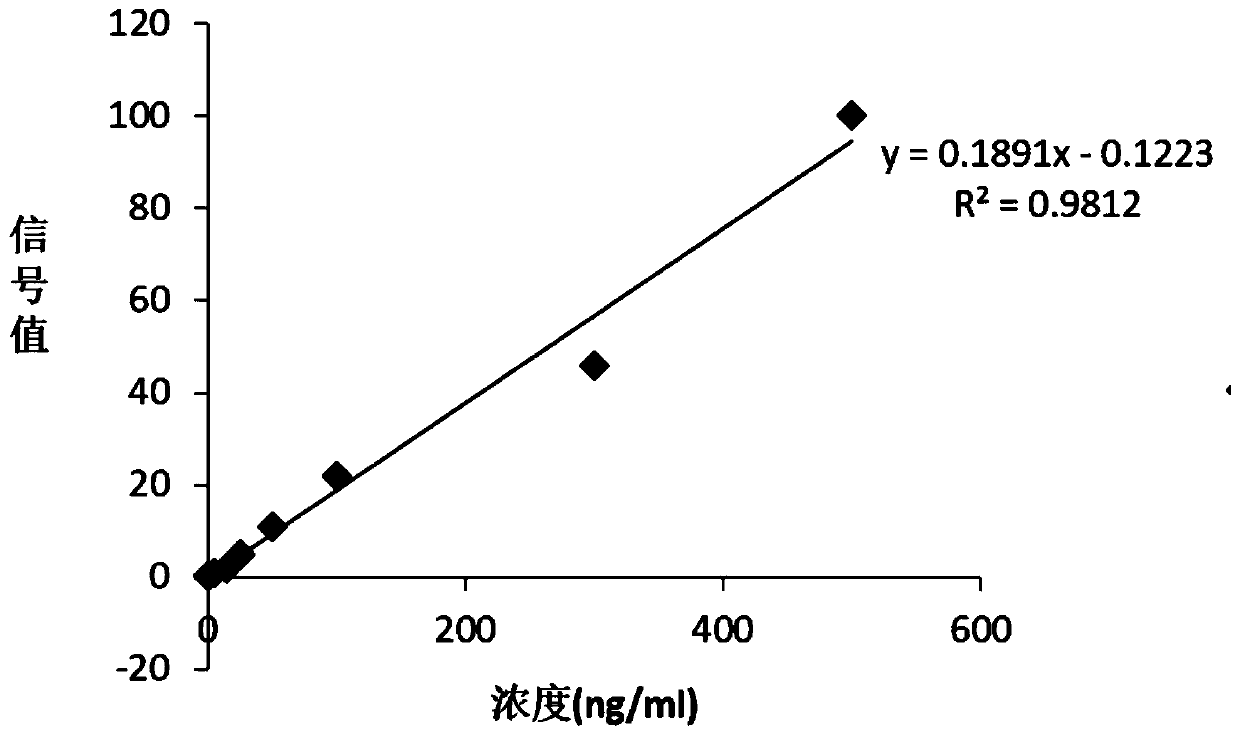
[0038] 1. How to use the kit
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com