Polymer containing DPP, thiophene and fluorothiophene structural units as well as preparation method and application of polymer
A technology of structural units and polymers, which is applied in the fields of electrical components, semiconductor/solid-state device manufacturing, and photovoltaic power generation. It can solve the problems of complex synthesis routes and raw materials, which need to be improved, and achieve strong promotion and application value, and a large molar absorptivity coefficient. , Optimize the effect of energy level
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0039] A preparation method of a polymer containing DPP, thiophene and fluorothiophene structural units, comprising the following steps:
[0040] Provide 2,5-dibromothiophene, 2,5-dibromo-3,4 difluorothiophene and compound DPP, the structural formula of compound DPP is:
[0041]
[0042] Among them, R is C 6 ~C 16 the alkyl group;
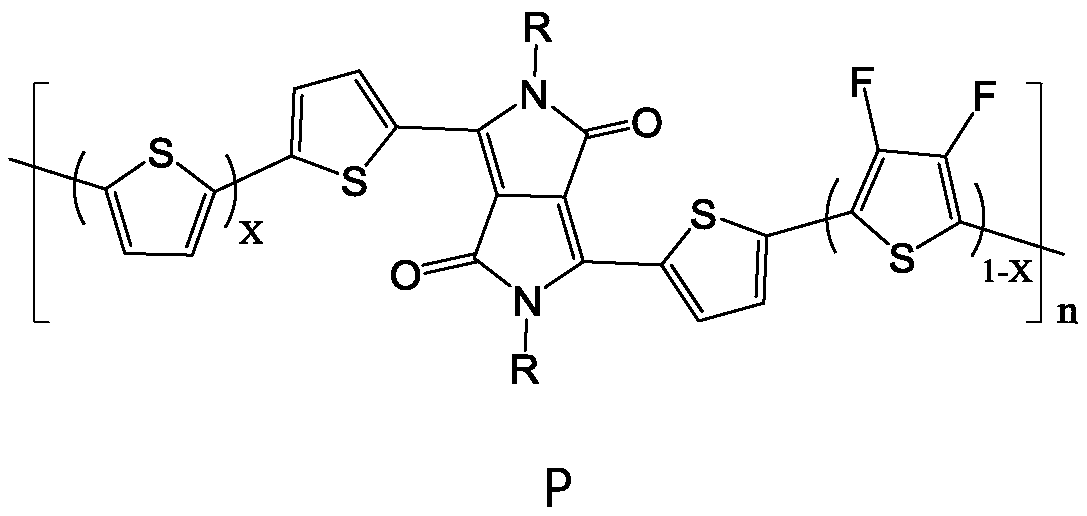
[0043] Under nitrogen protection conditions, according to the number of parts of the substance, (1.0 to 1.5) parts of compound R-DPP, a part of 2,5-dibromothiophene and b part of 2,5-dibromo-3,4 difluoro Add thiophene to an organic solvent, add a catalyst, and carry out a stille coupling reaction at 100°C to 130°C for 3 to 7 days; after separation and purification, a polymer containing DPP, thiophene and fluorothiophene structural units is obtained, and the DPP-containing The polymer of , thiophene and fluorothiophene structural unit is the polymer P with following general formula:
[0044]
[0045] Among them, 06 ~C 16 alkyl; n is an in...
Embodiment 1
[0056] Embodiment 1 2,5-dibromo-3, the synthesis of 4 difluorothiophene
[0057] Add LDA (273mmol) into a three-necked flask containing 150mL of anhydrous tetrahydrofuran to dissolve; after the solution is cooled to -78°C, slowly add nBuLi (273mmol), followed by 2,5-dibromothiophene (124mmol), The reaction was stirred for 2 h to obtain a yellow solution; after the reaction was completed, chlorotrimethylsilane (300 mmol) was added to the yellow solution within 1 h, then heated to room temperature and stirred overnight; the aqueous layer was removed with diethyl ether (100 mL), and the organic phase was It was dried over sodium sulfate, concentrated by filtration under reduced pressure, and vacuum distilled to obtain 3,4-dibromo-2,5-bis(trimethylsilyl)-thiophene with a yield of 90%.
[0058] Dissolve 3,4-dibromo-2,5-bis(trimethylsilyl)-thiophene (18mmol) in 80mL of anhydrous tetrahydrofuran, and after cooling to -78°C, add nBuLi (35mmol) and N-fluoro-N -(Benzenesulfonyl)benzene...
Embodiment 2
[0063] The synthesis of embodiment 2 polymer P1
[0064] 2,5-dibromothiophene (0.1mmol), 2,5-dibromo-3,4 difluorothiophene (0.1mmol) and compound C 6 h 13 -DPP (0.2mmol) was added to a 50ml flask, pumped and filled with nitrogen for 3 times, quickly added bistriphenylphosphine palladium dichloride (0.008mmol) catalyst in the state of bulging, and pumped and ventilated 3 times , add toluene solvent (40ml) and sodium carbonate (5ml), then the flask is heated to 120 ℃ to carry out Still coupling reaction 5 days, then cools down to stop polymerization reaction, then pours methanol (90ml) into the flask and settles, filters, and then Extracted with methanol and n-hexane for 24 hours, and finally extracted with chloroform, spin-dried, vacuum pumped and filtered overnight to obtain polymer P1 with a yield of 56%.
[0065] The specific synthetic route of polymer P1 is:
[0066]
[0067] After detection, the product polymer P1 Mn: 50.2kDa, PDI: 2.13.
[0068] The application of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com