Crystal form A of aminopyrimidine compound mesylate and preparation method and application thereof
An aminopyrimidine and mesylate technology, which is applied in the field of drug synthesis to achieve the effects of high bioavailability, good solubility and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
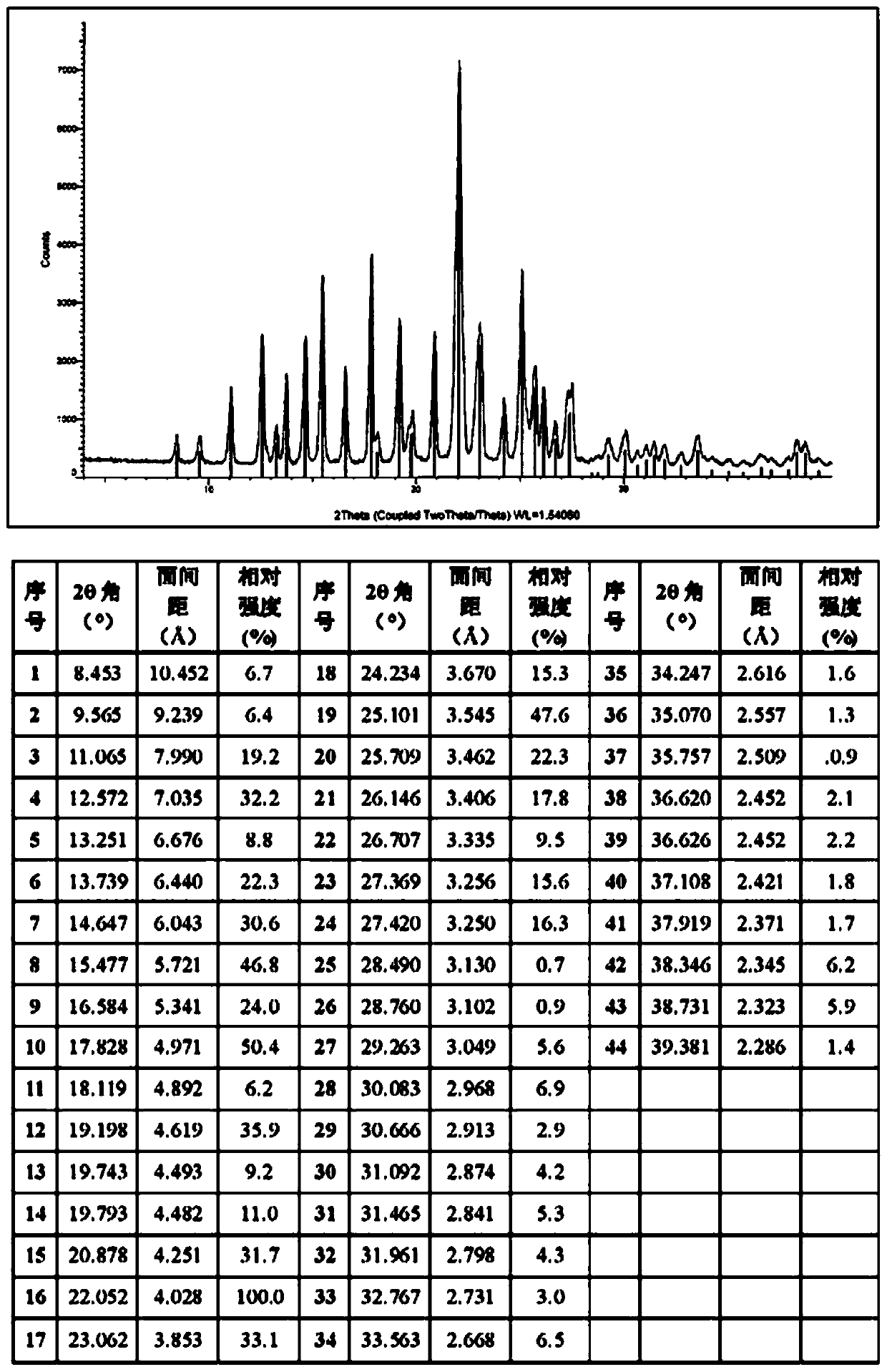
Image
Examples
preparation example Construction
[0041] Step 1 - Preparation of Intermediate J:
[0042]
[0043] Preparation: Add 6L of anhydrous tetrahydrofuran solvent to a 10L reaction flask, protect with nitrogen, and cool to 0°C. While stirring, 101 g of sodium hydrogen (101 g, 2.52 mol) was slowly added, and the internal temperature did not exceed 10° C., and 234 g of dimethylaminoethanol (234 g, 2.62 mol) was added. After the addition, the temperature was adjusted to room temperature to prepare a sodium alkoxide solution.
[0044] In the 30L reaction flask, add N-(4-fluoro-2-methoxy-5-nitrophenyl)-4-(1-methyl-1H-indol-3-yl)-2-pyrimidinamine ( Starting material B) (430g, 1.10mol), then add 9L of tetrahydrofuran, start stirring, dissolve clear, control the temperature at 10±10°C, and slowly add the prepared sodium alkoxide solution dropwise. Control the temperature at 10±10°C and keep it warm for 5.0h. When the raw material content is ≤0.5%, the reaction ends. Control the temperature at 10±10°C, slowly add 3% hy...
Embodiment 1
[0055]
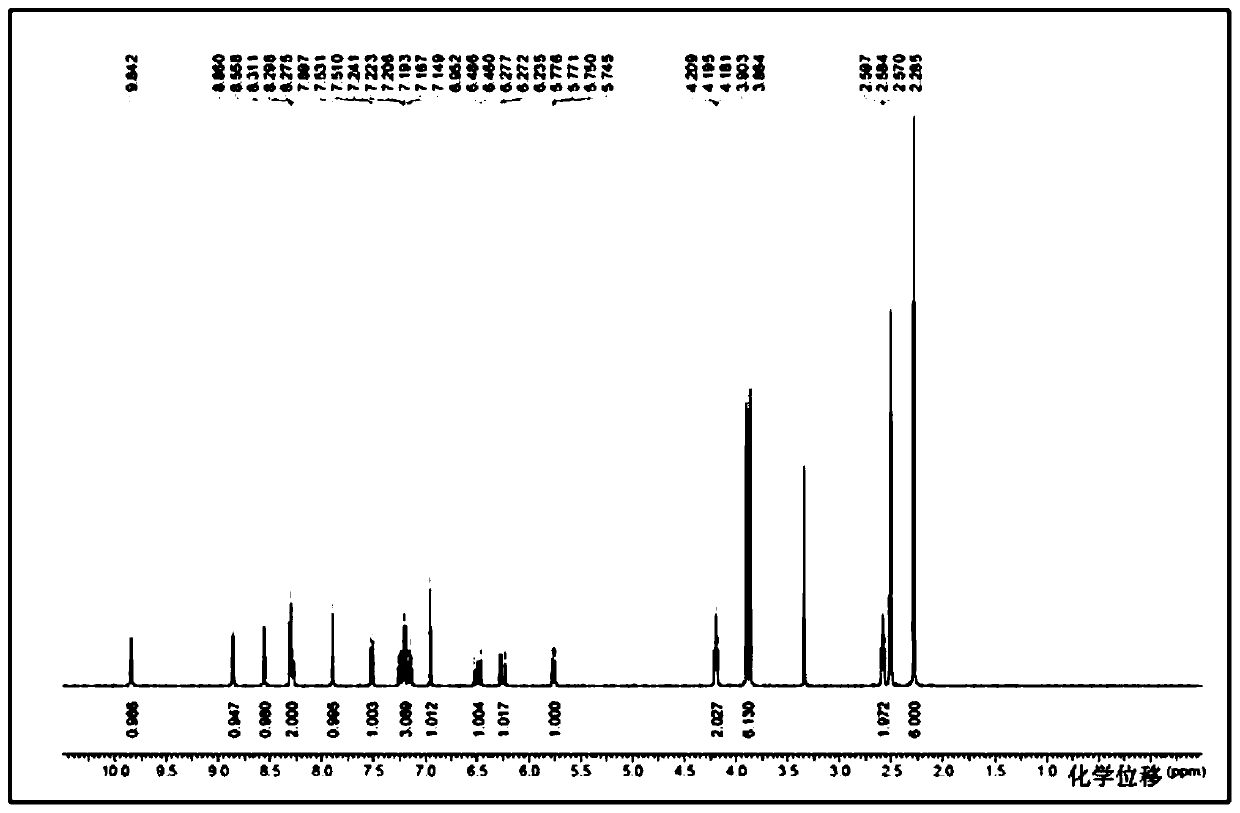
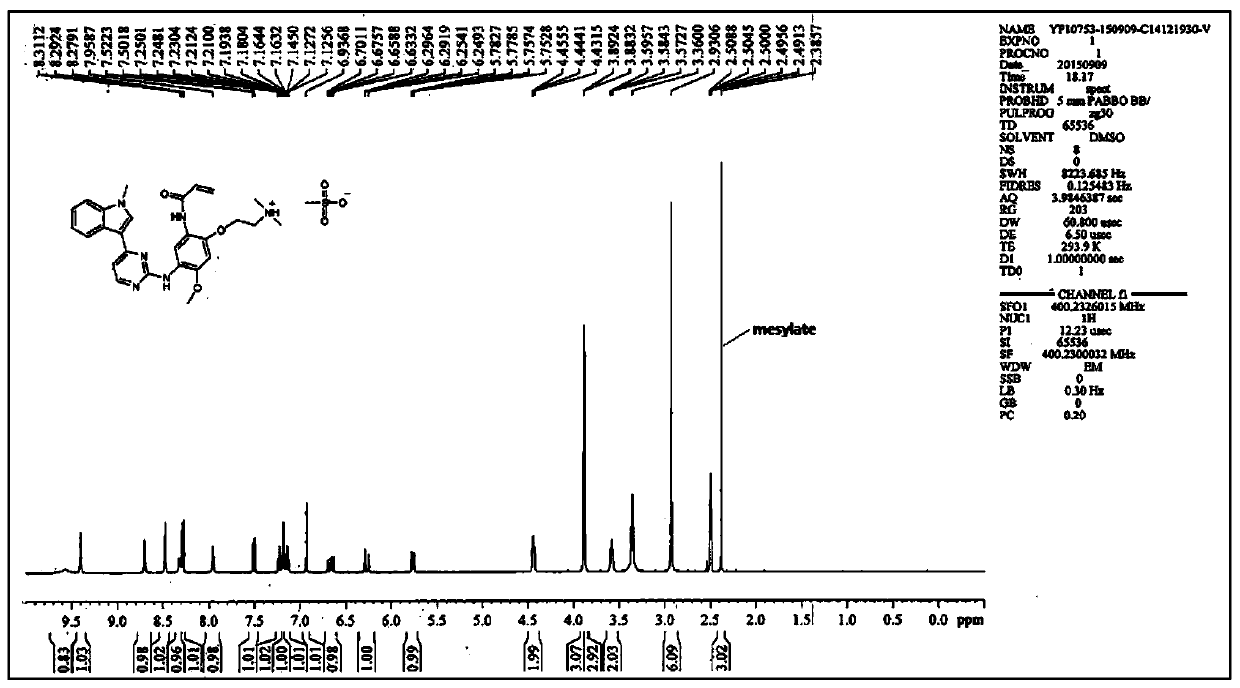
[0056] The compound of formula I (3 g, 6.1 mmol) was dissolved in 24 ml of dimethyl sulfoxide DMSO solvent, heated to 65 degrees, and stirred to dissolve. An equivalent of methanesulfonic acid (0.59 g, 6.1 mmol) was added to the system. Cool down to 50°C, and slowly add 12ml of isopropyl acetate IPAc. Stir at 50°C for 1 hour, then cool down to 15°C. 21 ml IPAc was added at 4 hours. The solution was stirred and crystallized at 15°C, filtered under reduced pressure, the filter cake was washed with isopropyl acetate, and washed with acetone to reduce the residual DMSO solvent. Air blow drying at 50°C (or vacuum drying at 50°C under reduced pressure) yielded 3.16 g of a light yellow solid (crystalline form A). HPLC purity 100%, yield 88%, DMSO:<100ppm; IPAc:<100ppm. MS m / z: 487.2 [M+1-MsOH]. Melting point: 242-244°C.
[0057] NMR data (attached figure 2 ): 1 HNMR (d6-DMSO): δppm: 9.57 (brs, 1H), 9.40 (s, 1H), 8.71 (s, 1H), 8.48 (s, 1H), 8.32 (d, 1H, J=7.9), 8....
Embodiment 2
[0060]
[0061] The compound of formula I (28.25 g, 58.1 mmol) was dissolved in 224 ml of dimethyl sulfoxide DMSO solvent, heated to 15-35 degrees, and stirred to dissolve. 0.97 equiv of methanesulfonic acid (5.4 g, 0.97 mmol) was added portionwise to the system. 448 ml methyl isobutyl ketone (MIBK) was added slowly. Stir for 1 hour, then cool down to 10-15 degrees. The solution forms a salt reaction at 10-15 degrees, takes a sample, and detects the residual compound of formula I in the mother liquor by HPLC (≤0.4%). After the reaction was completed, suction filtration under reduced pressure gave 32 g of the crude product of the compound mesylate of formula I.
[0062] Add 3 g of the crude mesylate salt of the compound of formula I into 24 ml of dimethyl sulfoxide DMSO solvent, stir to dissolve at 65 degrees, cool down, slowly add 48 ml of methyl isobutyl ketone (MIBK), and stir for crystallization 6-8 hours, suction filtration under reduced pressure, air blow drying at ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com