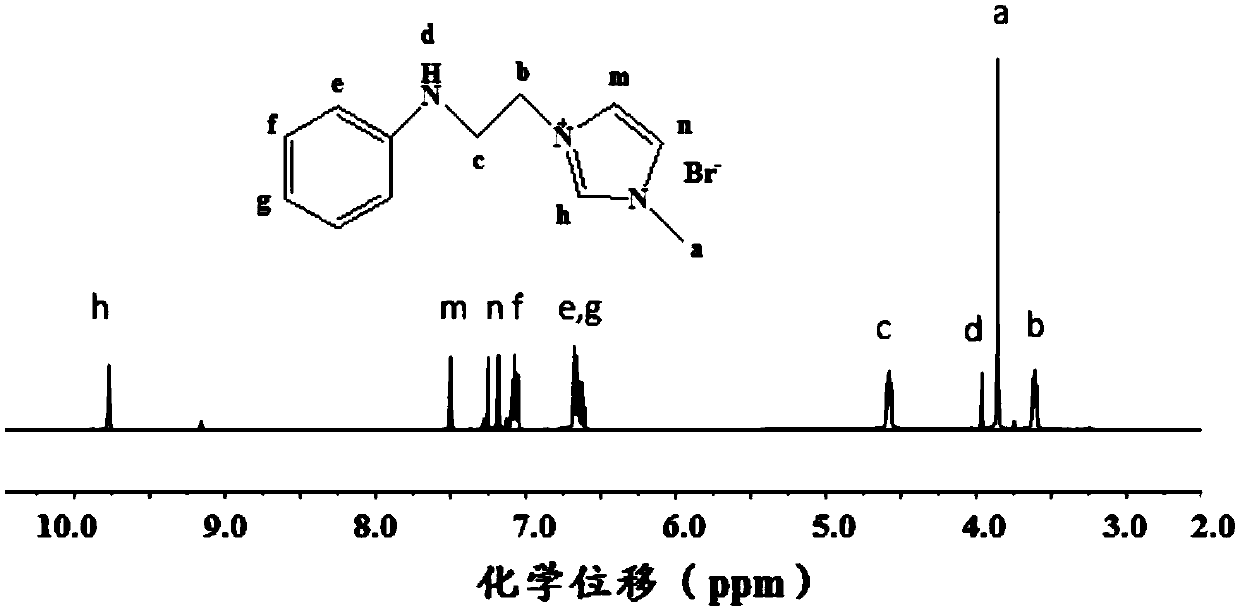
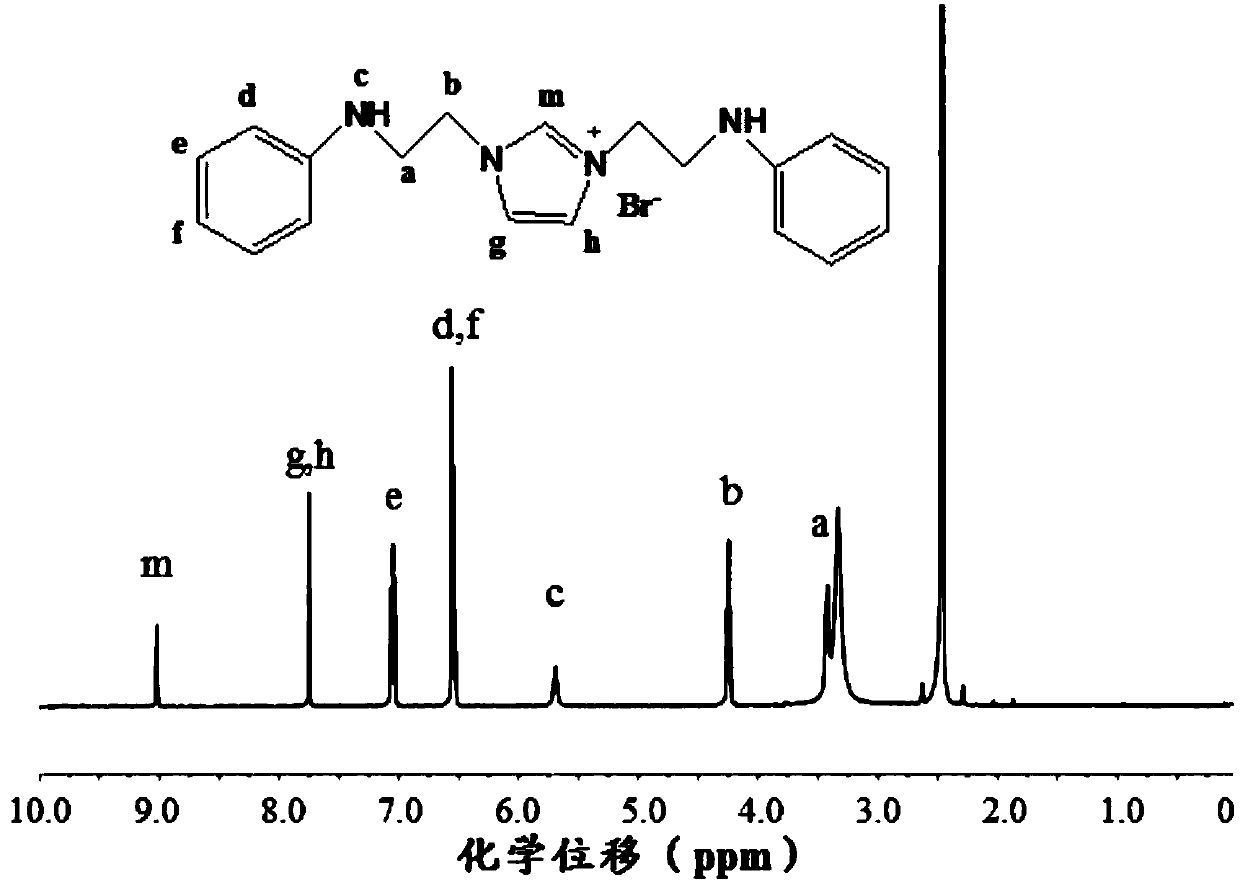
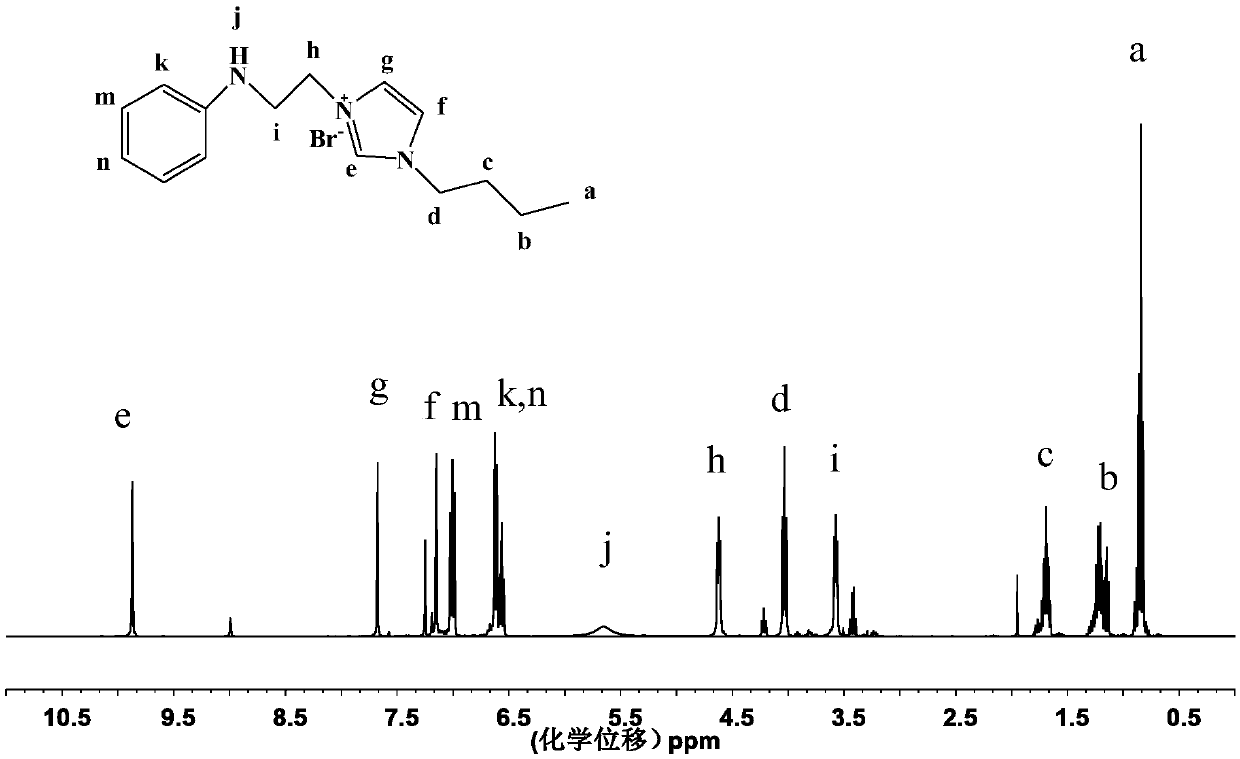
Cationic N-substituted aniline ionic liquid and preparation method thereof
An aniline and cationic technology, applied in organic chemistry and other fields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0041] According to a preferred embodiment of the present invention, step 1 includes the following sub-steps:
[0042] Step 1-1, preparing N-phenylethanolamine, and then adding hydrobromic acid at -5 to 5°C;
[0043] Step 1-2: Stir at low temperature (-10-10°C) for 20-40 minutes, then stir at room temperature (20-40°C) for 20-40 minutes to react;
[0044] Step 1-3, post-treatment after the reaction to obtain the N-phenylethanolamine hydrobromide.
[0045] In a further preferred embodiment, in step 1-1, the molar ratio of N-phenylethanolamine to hydrobromic acid is 1:(1-3), preferably 1:(1-2).
[0046] In a further preferred embodiment, in step 1-3, the post-treatment is carried out as follows: first rotary evaporation, redissolving, then performing water removal treatment and filtering, and finally rotary evaporation to remove the solvent.
[0047] According to a preferred embodiment of the present invention, step 2 includes the following sub-steps:
[0048] Step 2-1, addin...
Embodiment 1
[0074] Add 6.8780g of N-phenylethanolamine to a 100mL round bottom flask, add 8.1355g of hydrobromic acid at 0°C, stir in an ice-water bath for 30min, and then stir at room temperature for 30min. Remove water by rotary evaporation, add chloroform to dissolve and dry with anhydrous sodium sulfate, filter, and remove solvent by rotary evaporation to obtain light yellow N-phenylethanolamine hydrobromide solid. The yield was 86.3%.
[0075] Add 4.68mmol of N-phenylethanolamine hydrobromide into a two-neck flask, add 5mL of chloroform to dissolve, pass argon to remove oxygen, slowly drop 0.6mL of phosphorus tribromide into the flask, and stir in a water bath at 40°C for 5h. The reaction was quenched by adding 5 mL of deionized water, extracted three times with chloroform, and the organic phase was rotary evaporated to obtain N-phenylbromoethylamine hydrochloride as a white solid with a yield of 62.9%.
[0076] Dissolve N-phenylbromoethylamine hydrochloride in water, adjust the pH ...
Embodiment 2
[0080] Repeat the process of Example 1 to prepare N-phenylbromoethylamine.
[0081] Take 3.1011g of N-phenylbromoethylamine, 1.0560g of imidazole and 10mL of acetonitrile in a round bottom flask, and magnetically stir in an oil bath at 70°C for 5h. Precipitate with ether and wash with acetonitrile three times to obtain a white solid. Dry at 60°C under vacuum.
[0082] The NMR spectrum of the product is as figure 2 As shown, the test conditions: 1 H NMR, 400MHz, solvent is dimethyl sulfoxide (DMSO), obtains following result through analysis: the position of the hydrogen in the product structure is as follows figure 2 In the structural formula, the chemical shift δ of H at m is 9.03ppm (1H), the chemical shift δ of H at g and h is 7.76ppm (2H), and the chemical shift δ of H at e is 7.05ppm (4H), d , The chemical shift δ of H at f is 6.54ppm (6H), the chemical shift δ of H at b is 4.25ppm (4H), and the chemical shift δ of H at a is 3.37ppm (4H). The displacement of each pea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com