Industrial production method of 1, 4-dibromo-2, 5-diiodobenzene
A production method and technology of diiodobenzene, applied in the field of industrialized production of 1,4-dibromo-2,5-diiodobenzene, can solve the difficulty of post-processing, increase the risk of industrial production, the difficulty of post-processing, and the reaction temperature High-level problems, to avoid the use of concentrated sulfuric acid, to facilitate industrial production, the effect of mild synthesis conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] A kind of industrialized production method of 1,4-dibromo-2,5-diiodobenzene, specifically comprises the following steps:
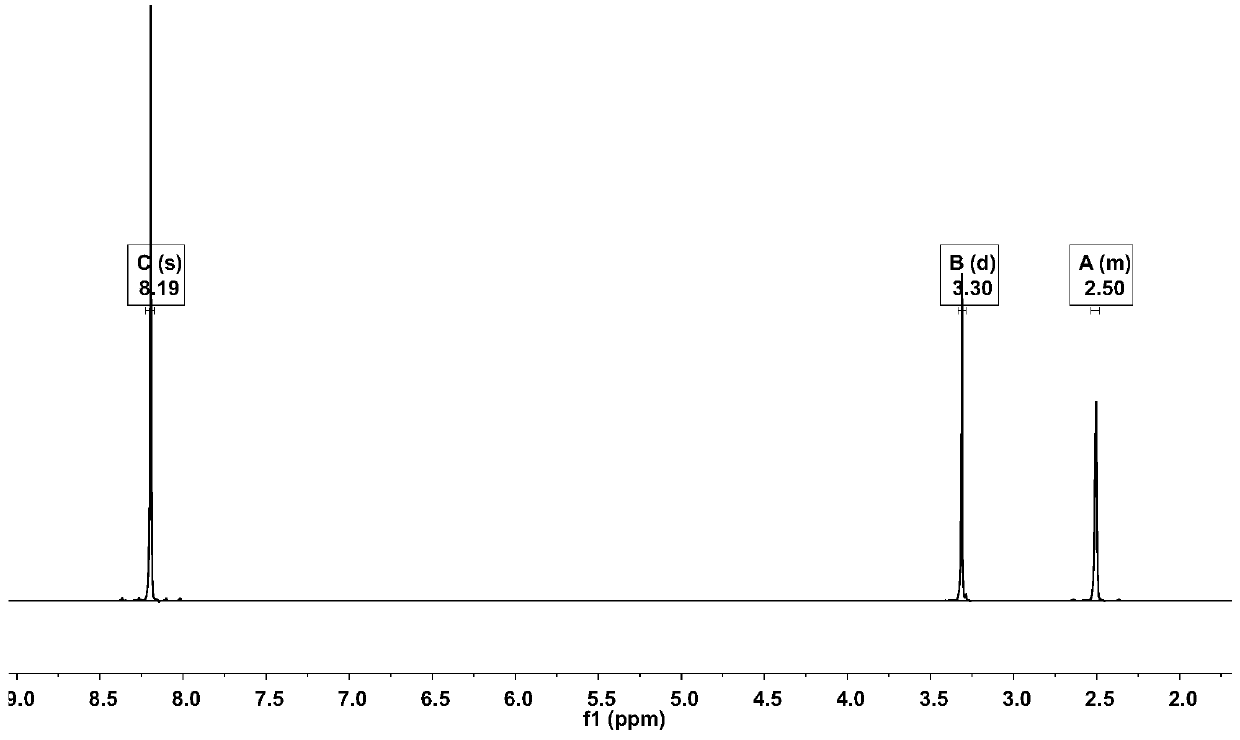
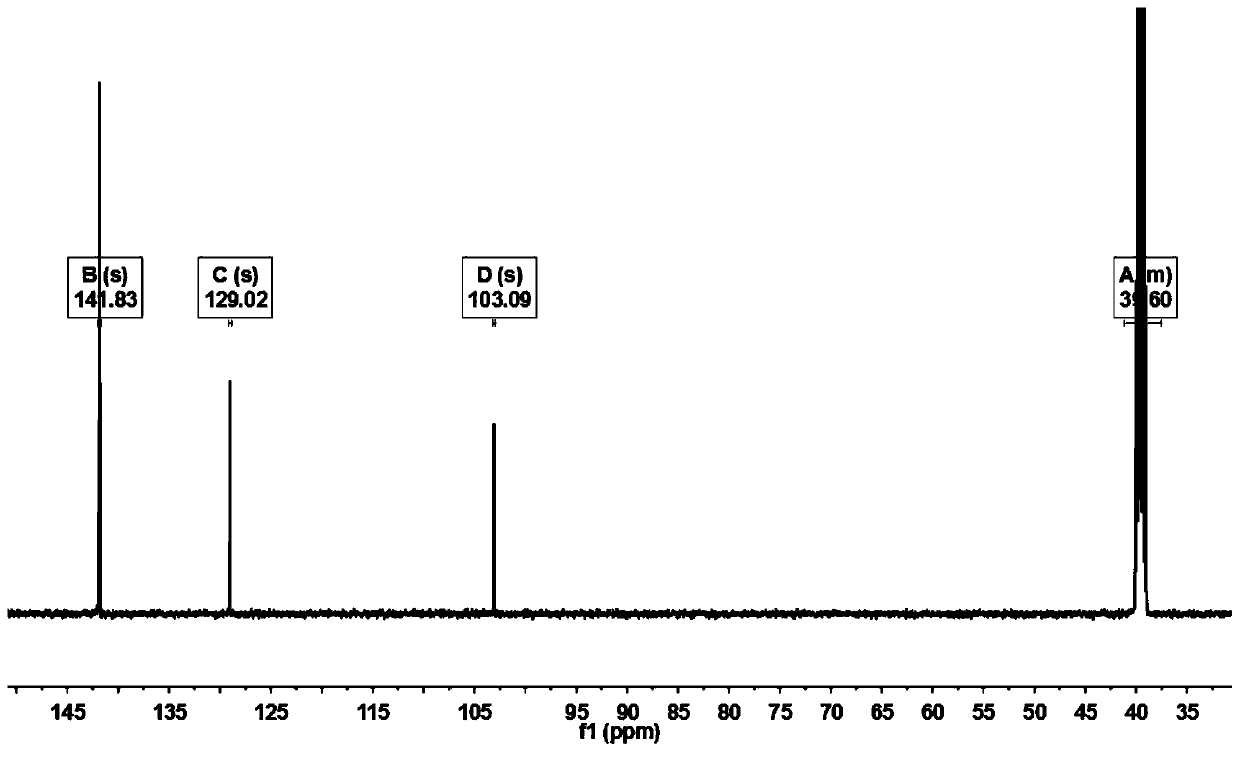
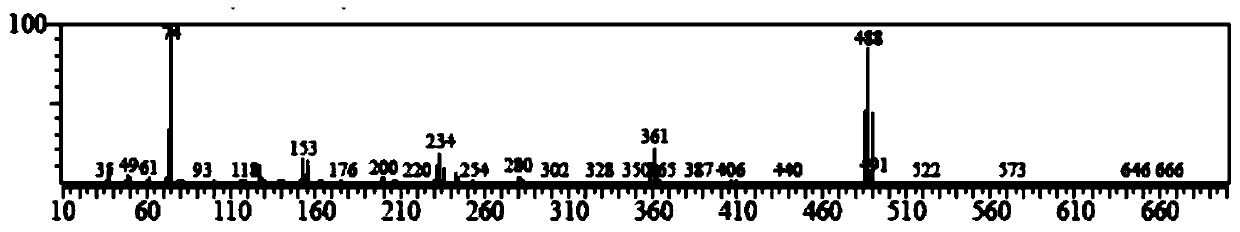
[0022] Add 3000mL of trifluoroacetic acid and 100.00g (423.89mmol) of 1,4-dibromobenzene to a 5000mL three-necked round-bottom flask in sequence, stir and raise the temperature to 68°C, and add 209.82g of N-iodosuccinimide in two times ( 932.56mmol), every interval of 2h, keep warm at 68°C for 20h, cool down to 20°C, add 3000mL of water to the system for dilution, filter, and recrystallize the filter cake with methylcyclohexane to obtain 148.39g of off-white solid, yield 71.78%. Such as Figure 1~3 as shown, 1 H NMR (500MHz, d 6 -DMSO): δ8.19(2H,s). 13 C NMR (500MHz, d 6 -DMSO): δ103.09, 129.02, 141.83. GC-MS: calcdfor C 6 h 2 Br 2 I 2 (M + ), 488; found, 488. This proves that 1,4-dibromo-2,5-diiodobenzene was successfully synthesized.
Embodiment 2
[0024] A kind of industrialized production method of 1,4-dibromo-2,5-diiodobenzene, specifically comprises the following steps:
[0025] Add 4000mL of trifluoroacetic acid and 100.00g (423.89mmol) of 1,4-dibromobenzene to a 5000mL three-necked round-bottom flask in sequence, stir and raise the temperature to 72°C, and add 190.73g of N-iodosuccinimide in two times ( 847.78mmol), every interval of 2h, keep warm at 72°C for 15h, cool down to 30°C, add 4000mL of water to the system for dilution, filter, and recrystallize the filter cake with methylcyclohexane to obtain 135.16g of off-white solid. The rate is 65.38%. GC-MS: calcd for C 6 h 2 Br 2 I 2 (M + ), 488; found, 488. 1 H NMR (500MHz, d 6 -DMSO): δ8.19(2H,s). 13 C NMR (500MHz, d 6 -DMSO): δ103.09, 129.02, 141.83.
Embodiment 3
[0027] A kind of industrialized production method of 1,4-dibromo-2,5-diiodobenzene, specifically comprises the following steps:
[0028] Add 2000mL of trifluoroacetic acid and 100.00g (423.89mmol) of 1,4-dibromobenzene to a 5000mL three-necked round-bottomed flask in sequence, stir and raise the temperature to 70°C, and add 247.95g of N-iodosuccinimide ( 1102.11mmol), every interval of 2h, keep warm at 70°C for 25h, cool down to 25°C, add 2000mL of water to the system for dilution, filter, and recrystallize the filter cake with methylcyclohexane to obtain 108.56g of off-white solid, yield 52.51%. 1 H NMR (500MHz, d 6 -DMSO): δ8.19(2H,s). 13 C NMR (500MHz, d 6 -DMSO): δ103.09, 129.02, 141.83. GC-MS: calcd for C 6 h 2 Br 2 I 2 (M + ), 488; found, 488.
[0029] Can obtain from above-mentioned Examples 1~3, compared with existing 1,4-dibromo-2,5-diiodobenzene synthetic method, the present invention adopts 1,4-dibromobenzene as raw material, in trifluoroacetic acid and Un...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com