Detection kit for novel coronavirus, influenza A and B and respiratory syncytial virus
A technology of syncytial virus and respiratory tract, which is applied in the fields of biomedicine and clinical diagnosis, can solve the problems of many co-infections of patients, adverse effects of new coronavirus prevention and control, and great hidden dangers of cross-infection, so as to reduce the burden on hospitals, false negatives and false negatives. Low positive, low false positive effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Example 1 Primer and probe design and screening
[0055] 1. The present invention analyzes several viral sequences and explores through a large number of studies, and designs primers and probe sets shown in Table 1-4:
[0056] Table 1 Combination of primers and probes of the present invention
[0057]
[0058] Table 2 Control group 1
[0059]
[0060] Table 3 Control group 2
[0061]
[0062] Table 4 Control group 3
[0063]
[0064] 2. At the same time, after optimization and exploration of the corresponding detection system, the following detection system was established: 23.5 μl RT-PCR amplification solution and 1.5 μl enzyme mixture were added to each PCR tube. And add 5 μl template RNA amount.
[0065] That is, the RT-PCR detection system (total volume 30μl) includes:
[0066] (1) 23.5 μl RT-PCR amplification solution, including primers, probes and PCR buffer;
[0067] The concentration of the probe used to detect the target gene was 200 nM, the c...
Embodiment 2
[0082] Example 2 kit
[0083] 1. New coronavirus, influenza A virus, influenza B virus and respiratory syncytial virus nucleic acid joint detection kit, including the following components:
[0084] (1) Primer and probe combination
[0085]By analyzing the DNA of clinical samples, the inventors obtained the primers and SEQ ID NOs of SEQ ID NO: 1~2, as shown in Table 1, which have complementary nucleotide sequences only to the 2019-nCoV ORF1ab of the new coronavirus, as shown in Table 1. The probe of ID NO: 3; the primer of SEQ ID NO: 4 ~ 5 and the probe of SEQ ID NO: 6 that are only complementary to the nucleotide sequence of the 2019-nCoV N target gene; only complementary to influenza A virus The nucleotide sequence of SEQ ID NO: 7 ~ 8 primers and the probe of SEQ ID NO: 9; only the primers and SEQ ID NO of the complementary nucleotide sequence of influenza B virus: 10 ~ 11 : the probe of 12; only with the primer of SEQ ID NO: 13~14 and the probe of SEQ ID NO: 15 of the comp...
Embodiment 3
[0111] Example 3 Detection example
[0112] 1. Experiment
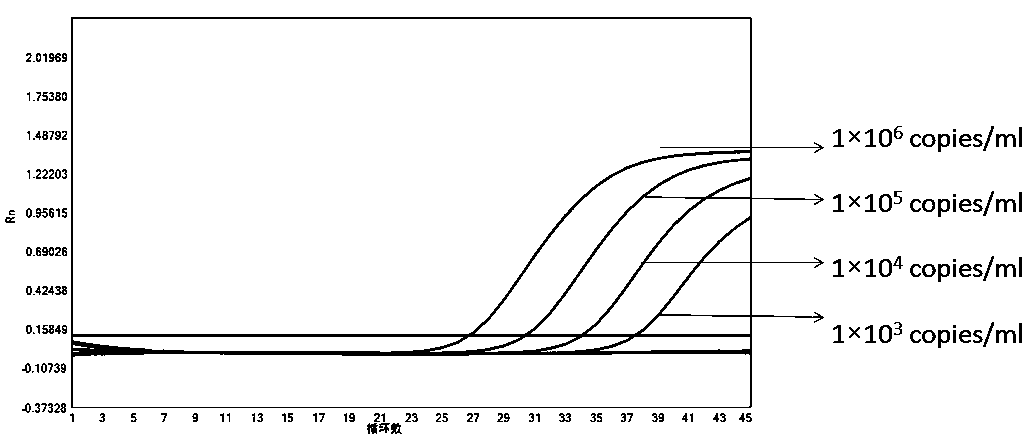
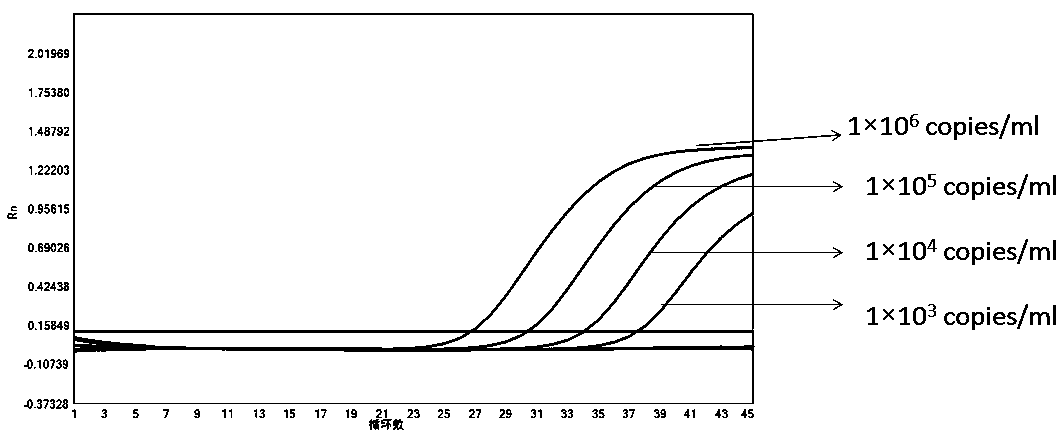
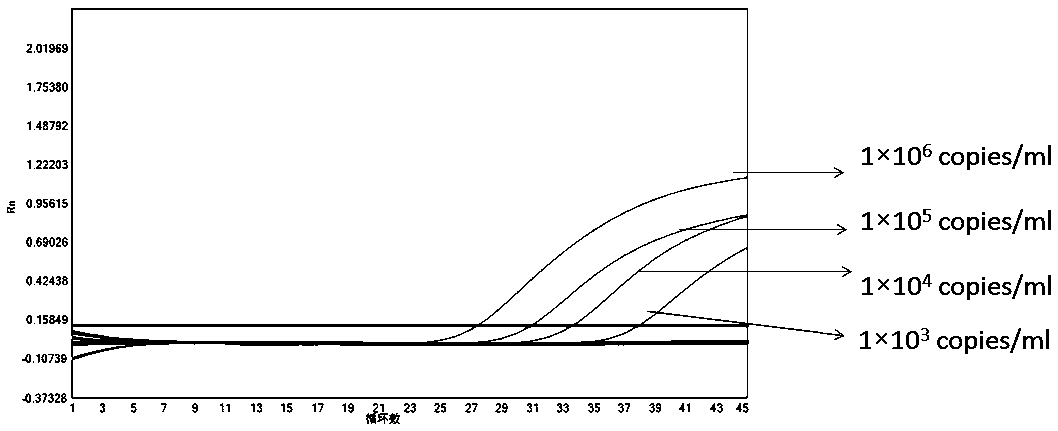
[0113] In order to test the accuracy and effectiveness of the kit of the present invention for the detection of the new coronavirus 2019-nCoV, influenza A, influenza B and respiratory syncytial virus, we tested the quality control products of the A-fluid plasmid, the B-fluid plasmid quality control, Respiratory syncytial virus plasmid quality control, new crown plasmid quality control, internal standard plasmid quality control (different concentrations: 1×10 6 copies / ml, 1×10 5 copies / ml, 1×10 4 copies / ml, 1×10 3 copies / ml, 1×10 2 copies / ml, 1×10 1 copies / ml) for testing, and Guangdong CDC new crown external quality assessment quality control product 5330 (dilute different concentrations: 1×10 5 copies / ml, 1×10 4 copies / ml, 1×10 3 copies / ml, 1×10 1 copies / ml), 18 cases of quality control products from Bondeson Company were tested.
[0114] 2. Results
[0115] The result is as Figure 1-6 The amplification ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com