Catalyst for preparing butadiene through oxidative dehydrogenation of butene and preparation method thereof
An oxidative dehydrogenation and catalyst technology, applied in the direction of carbon compound catalysts, physical/chemical process catalysts, catalysts, etc., can solve the problem of low selectivity of butadiene, and achieve the effects of high selectivity, stable performance and high stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
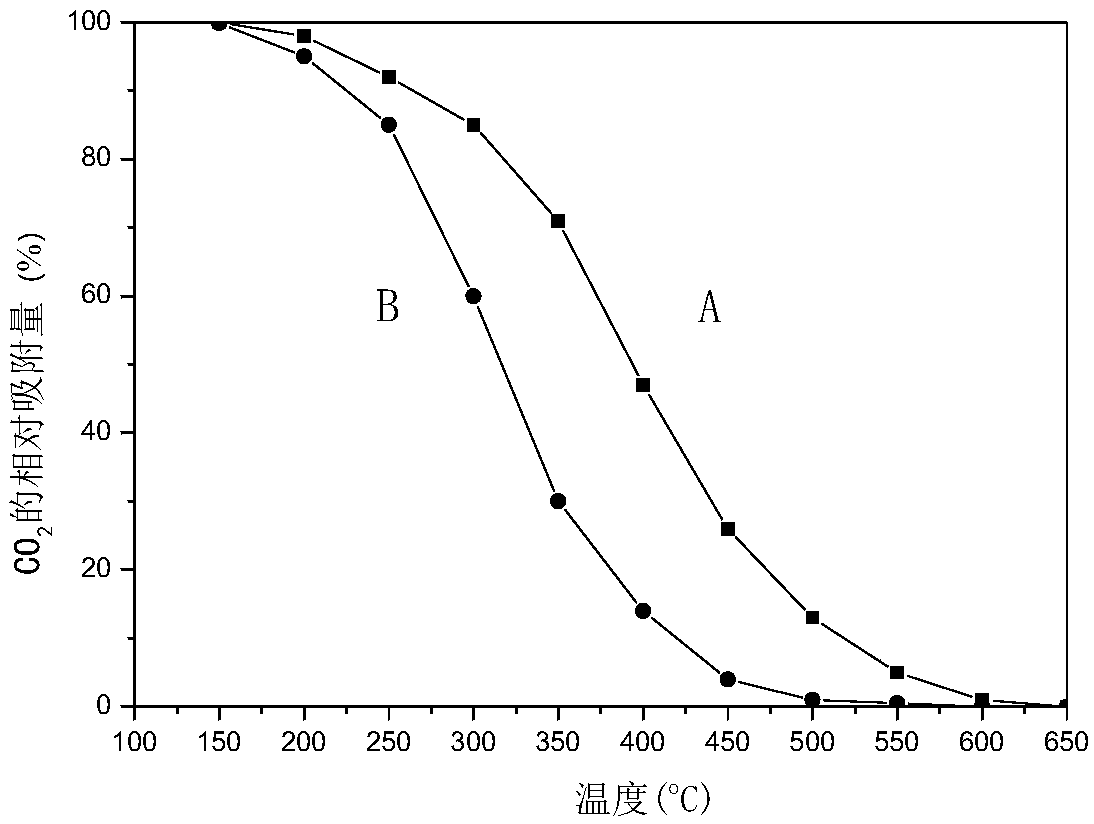
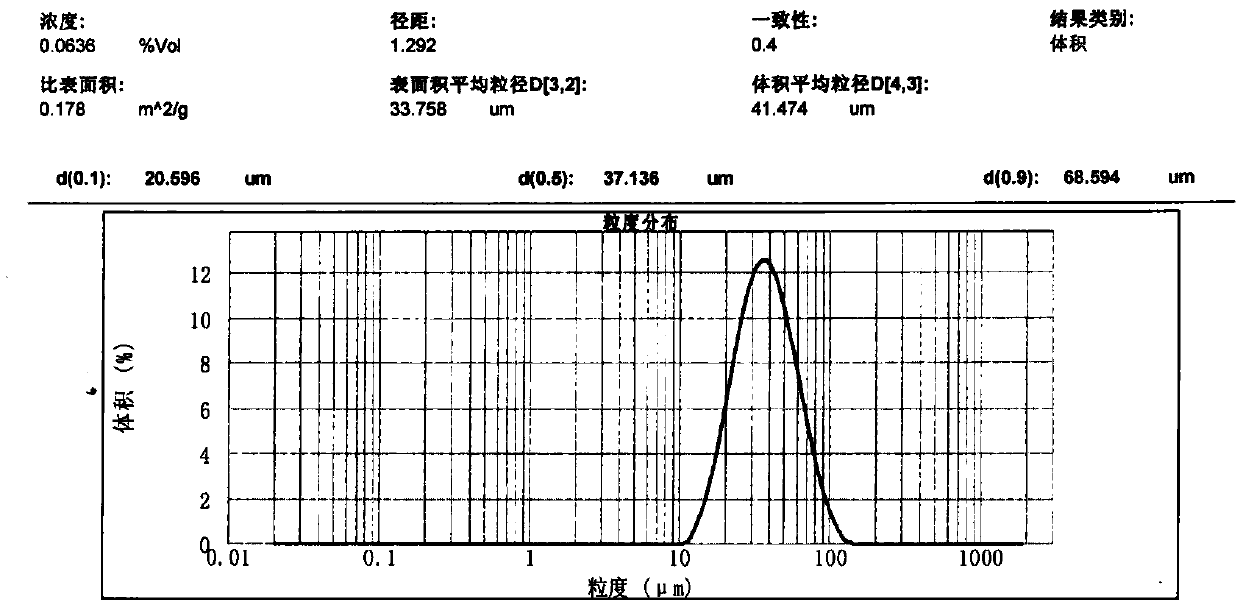
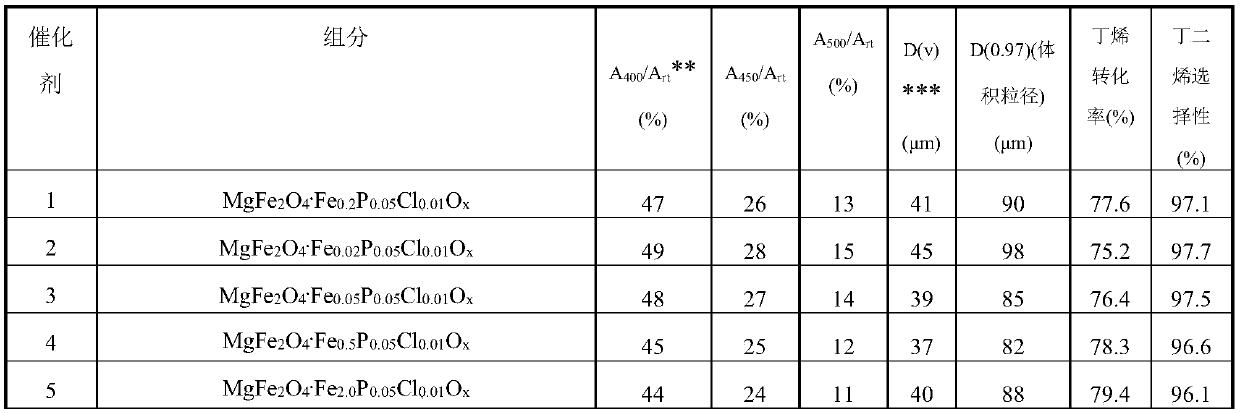
[0040] Weigh an appropriate amount of iron oxide red, MgO, MgCl 2 and magnesium phosphate, pulverized and sieved to obtain a catalyst precursor with a volume average particle diameter of 41 μm and a D(0.97) (volume particle diameter) of 90 μm. Add appropriate amount of graphite and water to the above mixture, stir in a kneader for 2 hours, take out the extruded strand, and dry the obtained solid in an oven at 110° C. for 4 hours. The dried samples were calcined in a muffle furnace at 700°C for 6 hours, and ground into 40-60 mesh particles for catalyst evaluation. Catalyst 1 at 400 °C CO 2 Adsorption capacity is room temperature CO 2 47% of saturated adsorption capacity at 450°C CO 2 Adsorption capacity is room temperature CO 2 26% of saturated adsorption capacity at 500°C CO 2 Adsorption capacity is room temperature CO 2 13% of the saturated adsorption capacity. The elemental composition of Catalyst 1 has a molar ratio of MgFe 2 o 4 .Fe 0.2 P 0.05 Cl 0.01 o x .
Embodiment 2
[0042] Weigh appropriate amount of iron oxide yellow, MgO, MgCl 2 and magnesium phosphate, pulverized and sieved to obtain a catalyst precursor with a volume average particle diameter of 45 μm and a D(0.97) (volume particle diameter) of 98 μm. The above mixture was added with an appropriate amount of kale powder and water, stirred in a kneader for 1 hour, taken out to extrude, and dried in an oven at 90°C for 24 hours. The dried samples were calcined at 460° C. for 24 hours in a muffle furnace, and ground into 40-60 mesh particles for catalyst evaluation. Catalyst 2 at 400 °C in CO 2 Adsorption capacity is room temperature CO 2 49% of saturated adsorption capacity at 450°C CO 2 Adsorption capacity is room temperature CO 2 28% of saturated adsorption capacity at 500°C CO 2 Adsorption capacity is room temperature CO 2 15% of the saturated adsorption capacity. The molar ratio of the element composition of catalyst 2 is MgFe 2 o 4 .Fe 0.02 P 0.05 Cl 0.01 o x .
Embodiment 3
[0044] Weigh an appropriate amount of iron oxide yellow, Mg(OH) 2 , MgCl 2 and magnesium phosphate, pulverized and sieved to obtain a catalyst precursor with a volume average particle diameter of 39 μm and a D(0.97) (volume particle diameter) of 85 μm. Add appropriate amount of polystyrene microspheres and water to the above mixture, stir in a kneader for 10 hours, take out and extrude, and dry the obtained solid in an oven at 150° C. for 1 hour. The dried samples were calcined at 850° C. for 1 hour in a muffle furnace, and ground into 40-60 mesh particles for catalyst evaluation. Catalyst 3 at 400 °C CO 2 Adsorption capacity is room temperature CO 2 48% of saturated adsorption capacity at 450°C CO 2 Adsorption capacity is room temperature CO 2 27% of saturated adsorption capacity at 500°C CO 2 Adsorption capacity is room temperature CO 2 14% of the saturated adsorption capacity. The molar ratio of the element composition of catalyst 3 is MgFe 2 o 4 .Fe 0.05 P 0.05...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com