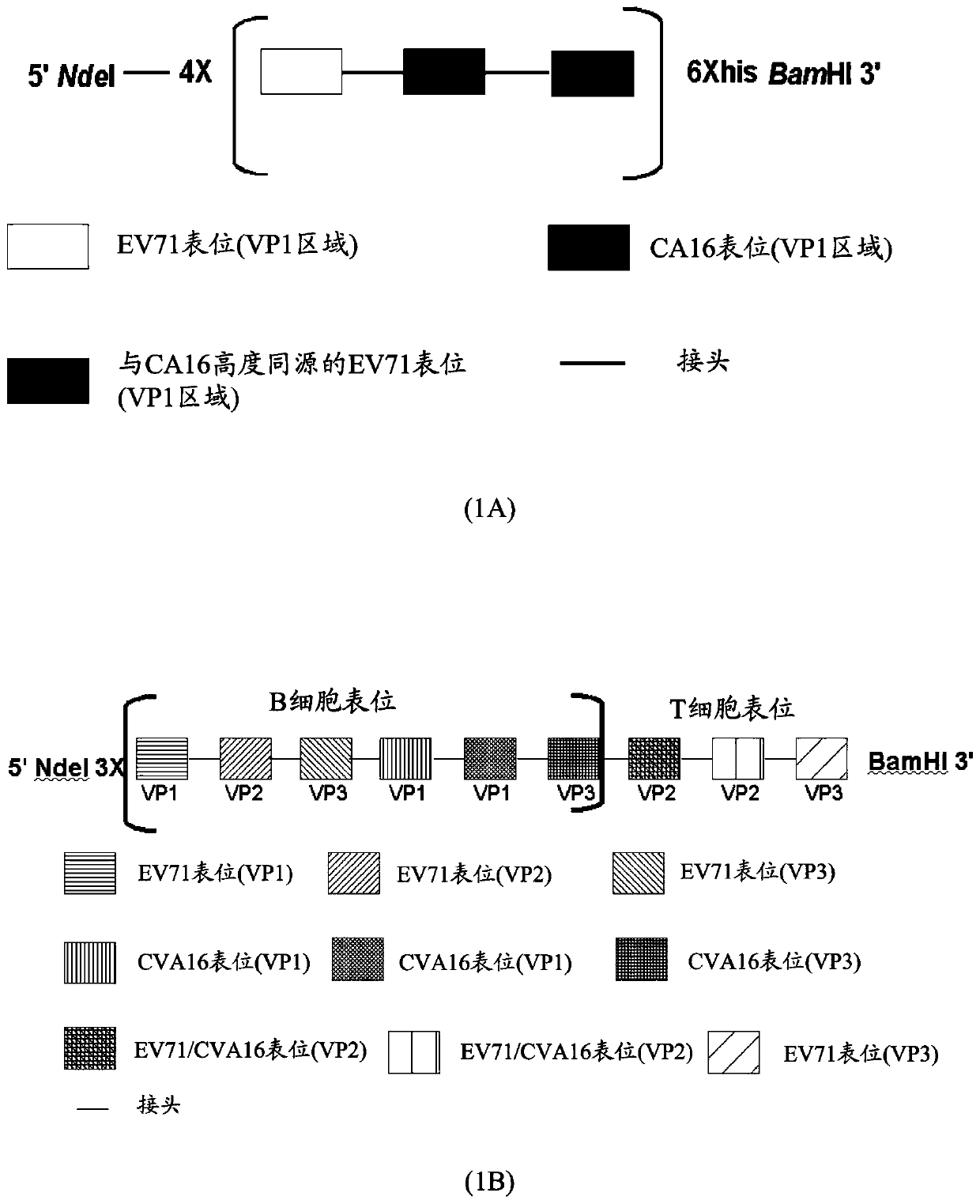
A synthetic polypeptide epitope based vaccine composition
A vaccine composition and vaccine antigen technology, which is applied in the field of vaccine compositions based on synthetic polypeptide epitopes, and can solve problems such as co-infection, destructive effects on medical systems, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Embodiment 1: express recombinant protein of the present invention by MEV1 and MEV2 gene construct
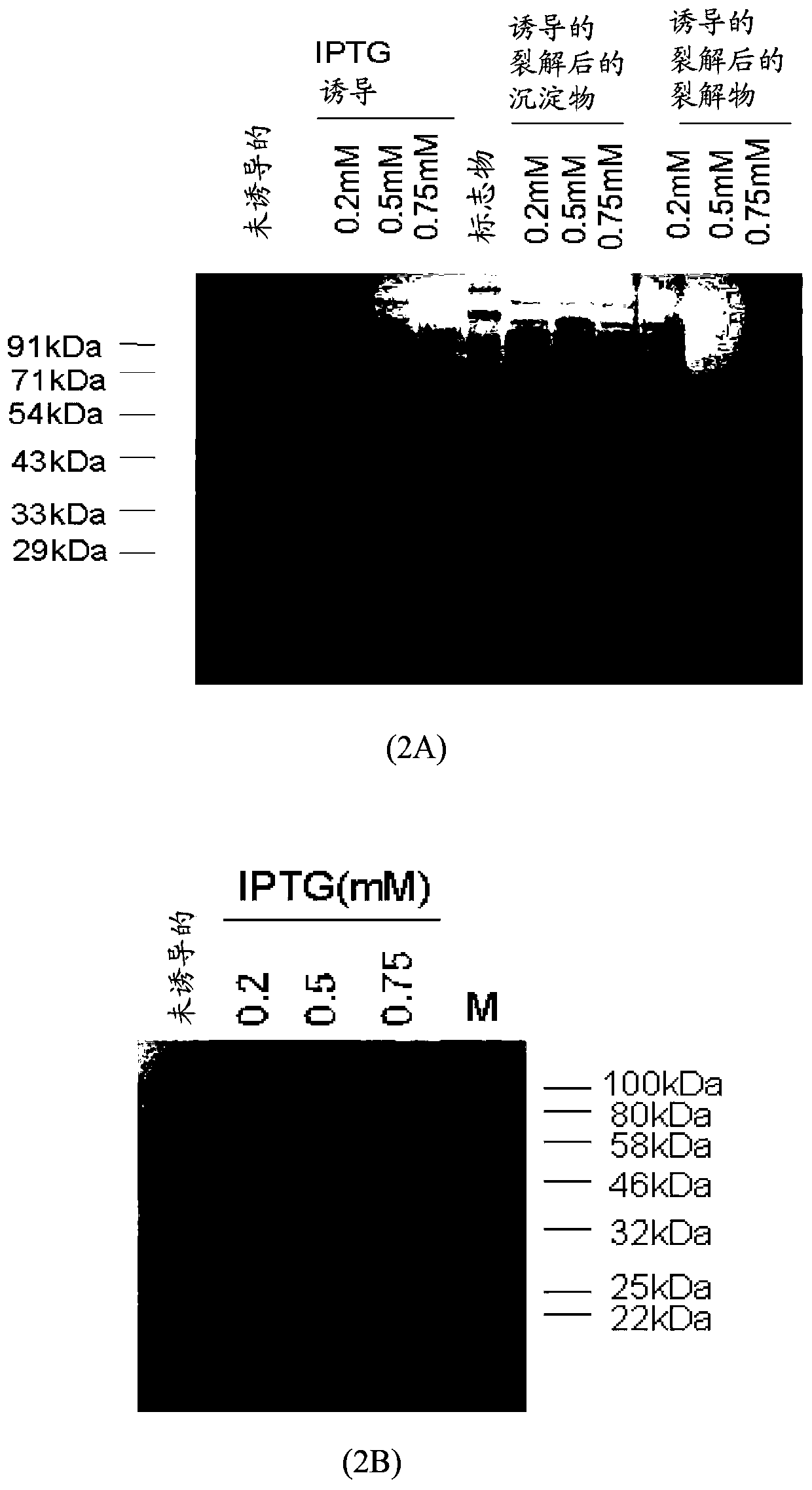
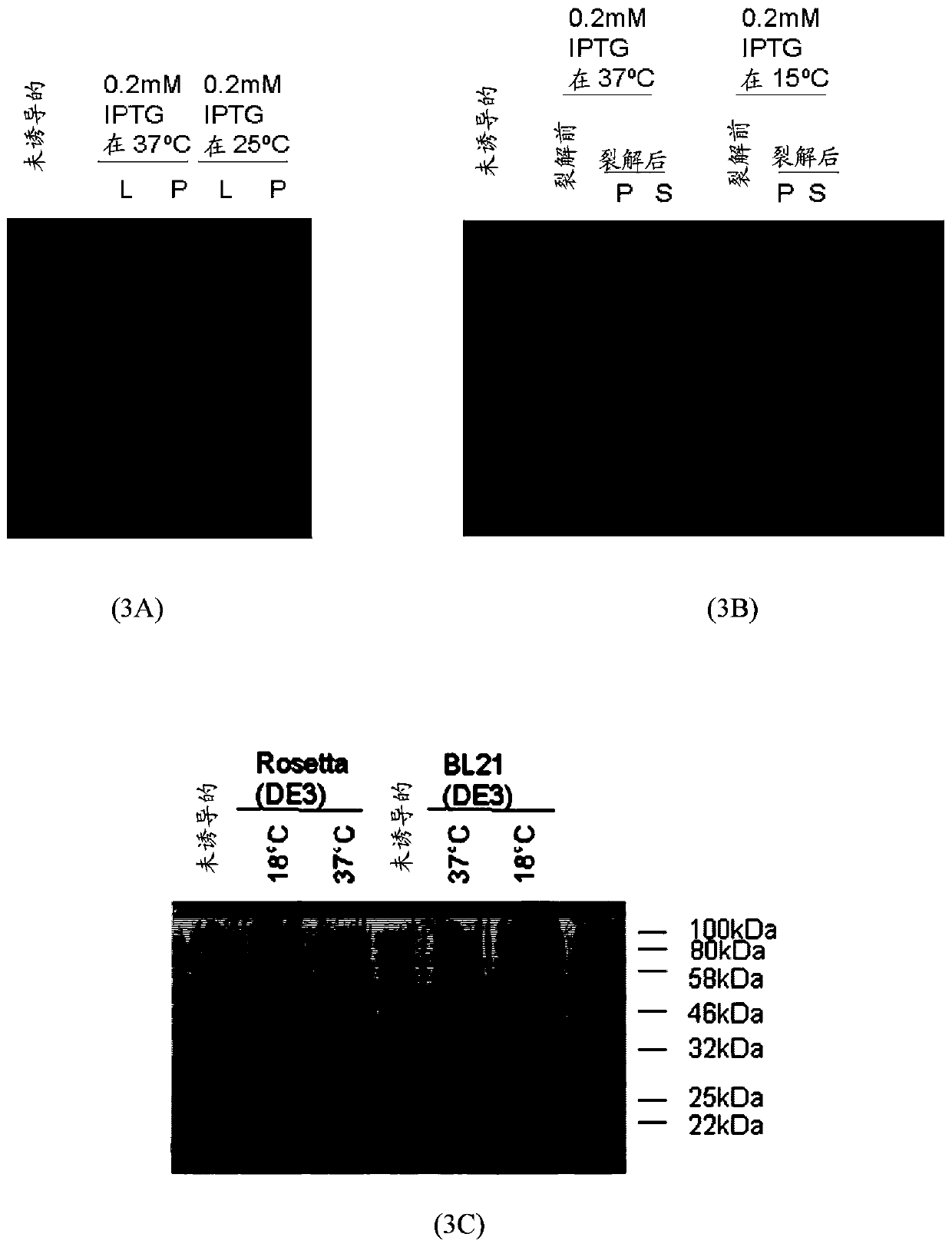
[0071] Plasmids pET11b and pET11b-MEV1 were transformed into E. coli BL21(DE3) chemically competent cells. Incubate transformed BL21(DE3) cells at 37°C with shaking until OD 600 0.4 to 0.6 were reached, and then induced with different concentrations of isopropyl β-D-1-thiogalactoside (IPTG) for 4 hours at 37°C. IPTG is a molecular mimic of lactose metabolites that triggers the transcription of the lac operon, and thus induces the expression of recombinant proteins whose genes are controlled by the lac operator. The optimal concentration of IPTG for expression was determined, and then induction of the multi-epitopic gene (MEV1) for expression was also determined in various temperature environments ( image 3 ). Optimal conditions for expression were evaluated for subsequent batches of experiments. pET11b-MEV2 was also transformed into BL21(DE3) cells, followed by incu...
Embodiment 2
[0072] Example 2: Cell Lysis with Lysis Buffer-I and Lysis Buffer-II
[0073] Cell pellets of MEV1 transformed BL21(DE3) after IPTG induction were collected by centrifuging the cells at 10,000 rpm for 5 min. Cells were suspended in Suspension / Lysis Buffer-I containing 50 mM Na 2 HPO 4 , 0.3M NaCl, 1% TritonX-114, 0.5mg / ml lysozyme, 1mM AEBSF, pH 7.4 solution to prepare. Cell lysis was performed first by performing three freeze-thaw cycles followed by three sonications of 20 seconds duration with 30 second intervals in between. The position of the expected protein was checked again by SDS-PAGE. Most of the expected protein was found in cellular debris, indicating the formation of insoluble / inclusion-like bodies. Then, the cells were lysed at 4°C for 3-16 hours in lysis buffer-II with different concentrations of urea (3M-6M) in the presence of 5mM-10mM imidazole, 50mM Na 2 HPO 4 , 0.3M NaCl and 1 mM AEBSF.
[0074] The results showed that the recombinant enterovirus vacci...
Embodiment 3
[0076] Example 3: Immobilized Metal Affinity Chromatography (IMAC) and Washing of MEV1 with Wash Buffer-I, Wash Buffer-II and Wash Buffer-III
[0077] The urea-denatured protein solution in the presence of 5 mM-10 mM imidazole was added to the Ni-NTA IMAC column after equilibration with Lysis Buffer-II and incubated at 4°C for 3-16 hours for proper binding. Then, the column was washed with Wash Buffer-I prepared by preparing a solution containing: 50 mM Na 2 HPO 4 , 0.3M NaCl, 1 mMAEBSF, 3M urea, pH 7.4, and 0.1% Triton X-114 and 5mM-10mM imidazole.
[0078] Perform the next wash in Wash Buffer-II prepared by making a solution containing: 50 mM Na 2 HPO 4 , 0.3M NaCl, 1 mM AEBSF, 3M-6M urea, pH 7.4, and 0.1% TritonX-114 and 20 mM imidazole. Subsequently, depending on the initial concentration of urea used in Example 2, at least two or more additional washes were performed with washing buffer-III in the presence of decreasing concentrations of urea through Prepared contain...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com