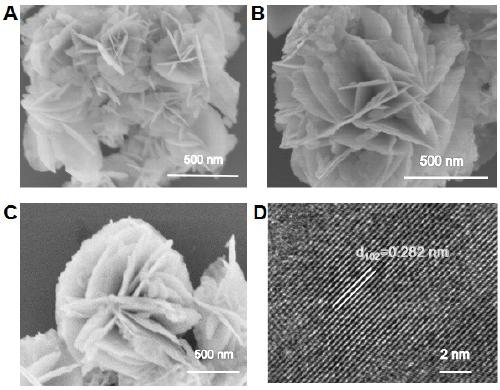
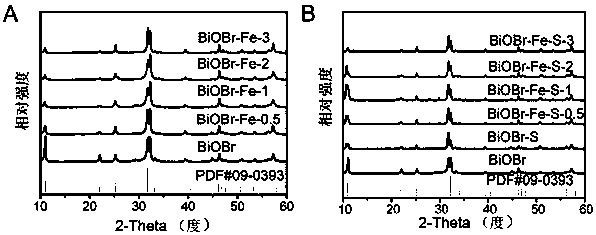
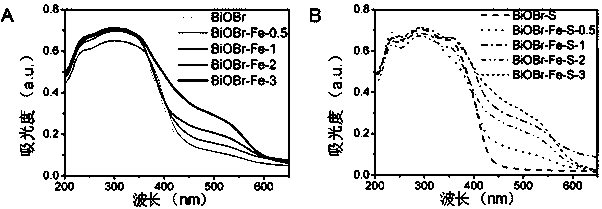
Oxygen vacancy-containing transition metal doped BiOBr nanosheet photocatalyst, preparation method and applications thereof
A transition metal and photocatalyst technology, applied in physical/chemical process catalysts, ammonia preparation/separation, chemical instruments and methods, etc., can solve problems such as restricting popularization and application, difficult adsorption and activation of inert nitrogen molecules, etc., to achieve environmental friendliness , Conducive to sustainable development, the effect of high catalytic efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Example 1 Preparation of BiOBr nanosheets:
[0023] Add 23.8 g / L potassium bromide solution dropwise to 48.5 g / L bismuth nitrate nitric acid solution in a volume ratio of 1:2, mix well and adjust the pH of the mixed solution to neutral with ammonia water, and add to the volume 100 mL Teflon-lined stainless steel autoclave. The autoclave was kept at 433 K for 18 hours and then cooled to room temperature. The precipitate was collected and washed with absolute ethanol and deionized water to remove organic solvent residues. The final product was dried in an oven at 333 K for 12 hours. Obtain BiOBr nanosheets.
Embodiment 2
[0024] Example 2 Preparation of BiOBr nanosheets doped with transition metals (iron, molybdenum, or nickel):
[0025] Add a solution of 0.08 M ferric chloride or ammonium molybdate or nickel chloride dihydrate to a 48.5 g / L bismuth nitrate nitric acid solution, and stir for ten minutes (the molar ratio of transition metal salt to bismuth nitrate is 0.5%). Add 23.8 g / L of potassium bromide solution to the above solution in a volume ratio of 1:2, mix evenly, adjust the pH of the mixed solution to neutral with ammonia, and add it to a 100 mL Teflon-lined stainless steel autoclave in. The autoclave was kept at 433 K for 18 hours and then cooled to room temperature. The precipitate was collected and washed with absolute ethanol and deionized water to remove organic solvent residues. The final product was dried in an oven at 333 K for 12 hours to obtain a transition metal-doped BiOBr catalyst product BiOBr-M-0.5 (M = Fe, Mo or Ni) without oxygen vacancies.
Embodiment 3
[0026] Example 3 Preparation of transition metal (iron, molybdenum, or nickel) doped BiOBr nanosheets:
[0027] Add a solution of 0.08 M ferric chloride or ammonium molybdate or nickel chloride dihydrate to a 48.5 g / L bismuth nitrate nitric acid solution, and stir for ten minutes (the molar ratio of metal salt to bismuth nitrate is 1%). Add 23.8 g / L of potassium bromide solution to the above solution in a volume ratio of 1:2, mix evenly, adjust the pH of the mixed solution to neutral with ammonia, and add it to a 100 mL Teflon-lined stainless steel autoclave in. The autoclave was kept at 433 K for 18 hours and then cooled to room temperature. The precipitate was collected and washed with absolute ethanol and deionized water to remove organic solvent residues. The final product was dried in an oven at 333 K for 12 hours to obtain a transition metal-doped BiOBr catalyst product BiOBr-M-1, (M = Fe / Mo / Ni) without oxygen vacancies.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com