Synthesis method of rucaparib camsylate
A technology of camphorsulfonate and synthesis method, which is applied to the preparation of sulfonic acid, chemical instruments and methods, and the preparation of organic compounds, etc., which can solve the problems of not being able to meet the requirements of medicine, unqualified refined products, and low utilization rate of raw materials. Achieve the effect of improving synthesis efficiency, high utilization rate and short reaction cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
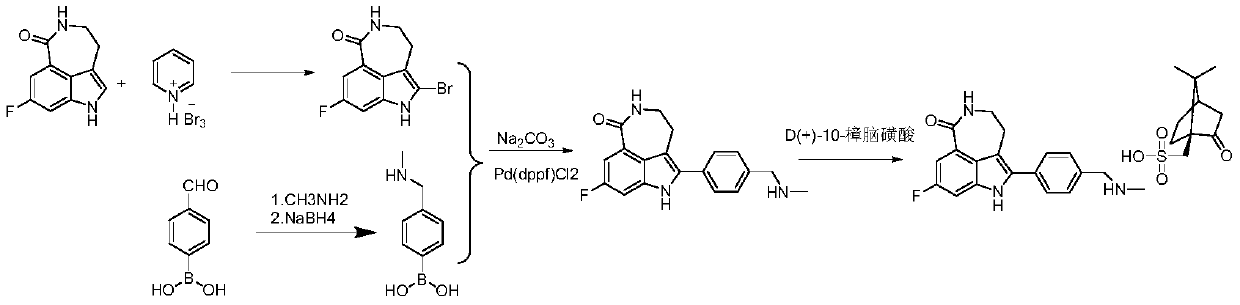
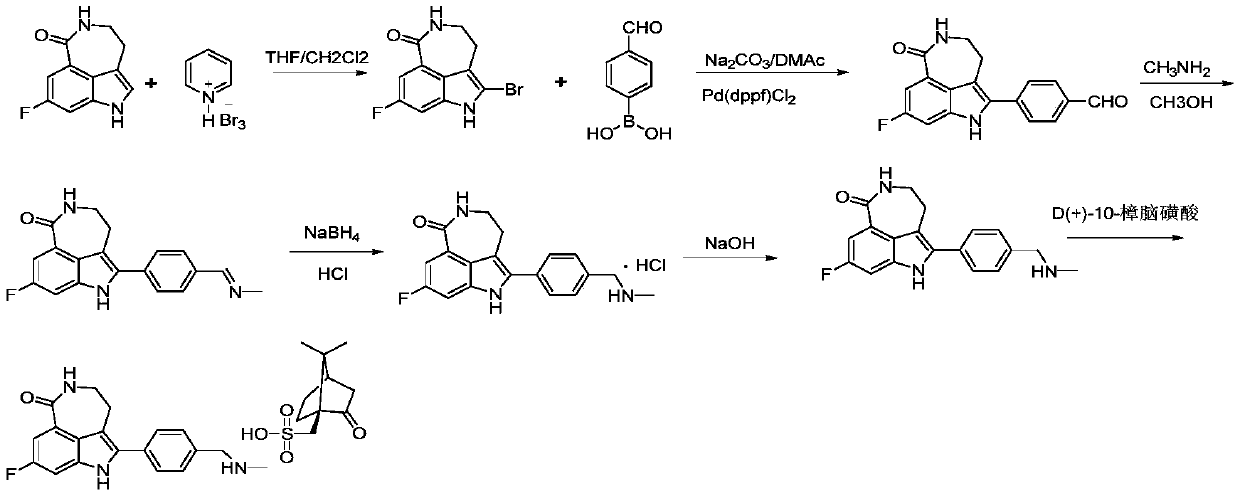
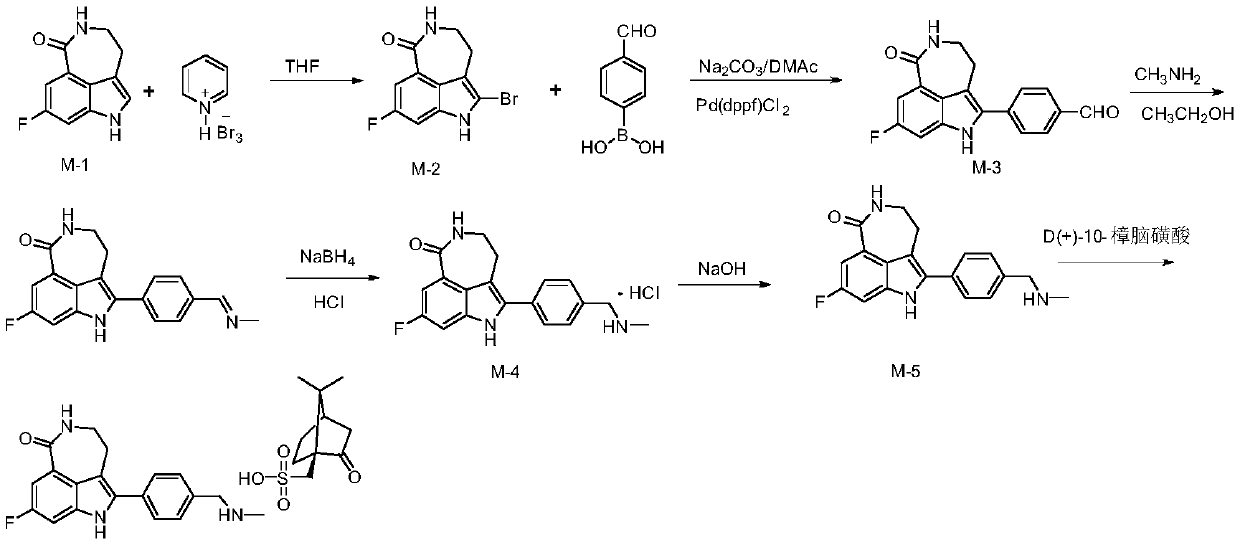
[0036] Step 1: Take compound M-1 (80g, 0.392mol) and add it to 600mL tetrahydrofuran, then add pyridinium tribromide (150.36g, 0.468mol), react at 10°C for 1h and filter with suction, add the filter cake to After stirring in 5% aqueous sodium bicarbonate solution (400 mL) for 1 h, suction filtration, the filter cake was washed with water (200 mL), and air-dried to obtain pale yellow compound M-2 (100.54 g), with a yield of 90.65%.
[0037] Step 2: Take compound M-2 (90g, 0.318mol), add it to a mixed solution of 900mL N,N-dimethylacetamide and 135mL water, add p-formylphenylboronic acid (57.20g, 0.382mol), sodium carbonate (84.24g, 0.795mol) and Pd(dppf)Cl 2 (6.98g, 9.54mmol), heated up to 90-100°C and reacted for 2h (this process was carried out under nitrogen protection), after the reaction, add water (1800mL) and crystallize at 10°C for 1h, then suction filter, and wash the filter cake with water to neutral properties, dried, added to absolute ethanol (720mL), stirred at 7...
Embodiment 2
[0042]Step 1: Take compound M-1 (80g, 0.39mol) and add it to 600mL tetrahydrofuran, then add pyridinium tribromide (150.36g, 0.468mol), react at 20°C for 1h and filter with suction, add the filter cake to After stirring in 5% aqueous sodium bicarbonate solution for 1 h, suction filtration, the filter cake was washed with water until neutral, and air-dried to obtain light yellow compound M-2 (101.29 g), with a yield of 91.23%.
[0043] Step 2: Take compound M-2 (90g, 0.318mol), add it to a mixed solution of 900mL N,N-dimethylacetamide and 135mL water, add p-formylphenylboronic acid (57.20g, 0.382mol), sodium carbonate (84.24g, 0.795mol) and Pd(dppf)Cl 2 (6.98g, 9.54mmol), heated to 90-100°C and reacted for 2h (this process was carried out under the protection of nitrogen), after the reaction, add water (1800mL), crystallize at 10°C for 1h and suction filter, the filter cake was washed with water until Neutral, dry, add absolute ethanol (720mL), stir at 75-80°C for 1h, drop to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com