Novel preparation method of nicergoline
A technology of nicergoline and ergot alcohol, which is applied in the field of new preparation of nicergoline, can solve the problems of unfavorable industrial production, difficult recycling, easy decomposition, etc., to avoid harmful substances and useless by-products, easy recycling, recycling high rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
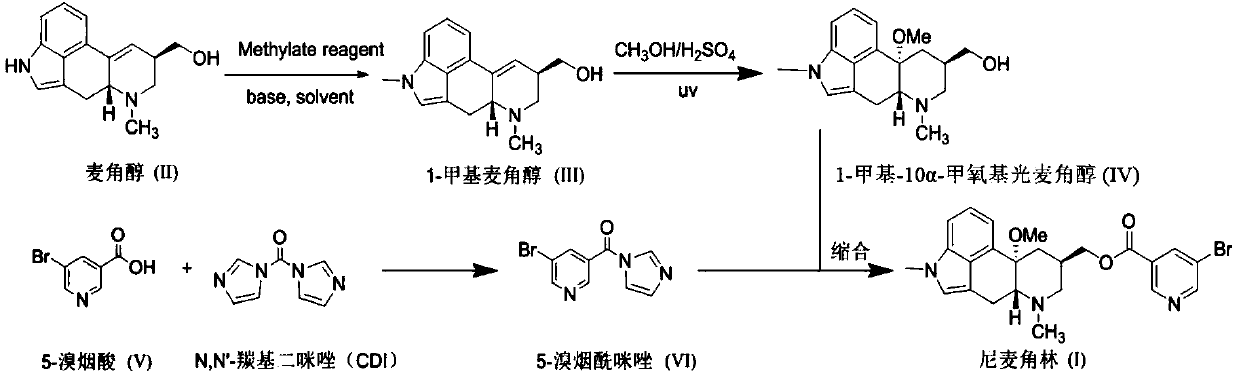
[0049] Dissolve ergot alcohol (50.8g, 0.2mol) in dimethyl sulfoxide (150mL), add potassium hydroxide (11.2g, 0.2mol), stir at room temperature for 45 minutes, then add dropwise methyl iodide (28.4g, 0.2mol) The dimethyl sulfoxide (50mL) solution was added dropwise in about 1h, and continued for 1h after the addition. TLC detection (UV254, methanol / chloroform / ammonia = 1:8:0.1) had a complete reaction, and the reaction solution was poured into ice water. The solid precipitated, was filtered and washed with water, and the solid was recrystallized with methanol, filtered, and dried to obtain 41.3 g of 1-methylergot alcohol with a HPLC purity of 99.1%.
[0050] Add 1-methylergot alcohol (40.2g, 0.15mol) into a pre-prepared mixed solution of 98% sulfuric acid (15.0g, 0.15mol) anhydrous methanol (500mL), cool the reaction solution to 0-5°C, and turn on UV lamp irradiation (wavelength 254nm, power 250W), control internal temperature 0-5 ℃ stirring reaction for 48 hours, TLC detection...
Embodiment 2
[0054] Dissolve ergot alcohol (50.8g, 0.2mol) in N,N-dimethylformamide (150mL), add sodium hydroxide (9.6g, 0.24mol), stir at room temperature for 45 minutes, then add dimethyl sulfate dropwise (27.7g, 0.22mol) of N,N-dimethylformamide (50mL) solution, the dropwise addition was completed in about 1h, and continued for 1h after the addition, and TLC detection (UV254, methanol / chloroform / ammonia water=1:8:0.1) After the reaction was complete, the reaction solution was poured into ice water, and solids were precipitated, filtered and washed with water, and the solids were slurried with methanol, filtered, and dried to obtain 45.2 g of 1-methylergodol with an HPLC purity of 99.5%.
[0055] Add 1-methylergot alcohol (40.2g, 0.15mol) into the pre-configured 98% sulfuric acid (45.0g, 0.45mol) mixed solution of anhydrous methanol (750mL), turn on the ultraviolet lamp irradiation (wavelength 365nm, power 750W) , control the internal temperature at 10-20°C and stir for 12 hours, TLC det...
Embodiment 3
[0058] Dissolve ergot alcohol (50.8g, 0.2mol) in N,N-dimethylacetamide (150mL), add potassium hydroxide (16.8g, 0.3mol), stir at room temperature for 45 minutes, then add dropwise dimethyl carbonate (21.6g, 0.24mol) of N,N-dimethylacetamide (50mL) solution, the dropwise addition was completed in about 1h, and the addition was continued for 1h, and TLC detection (UV254, methanol / chloroform / ammonia water=1:8:0.1) After the reaction was complete, the reaction solution was poured into ice water, and solids were precipitated, filtered and washed with water, and the solids were washed with a small amount of methanol-water, filtered, and dried to obtain 50.1 g of 1-methylergodol with a HPLC purity of 98.5%.
[0059] Add 1-methylergot alcohol (40.2g, 0.15mol) into the pre-configured 98% sulfuric acid (75.0g, 0.75mol) anhydrous methanol (750mL) mixed solution, turn on the ultraviolet lamp irradiation (wavelength 365nm, power 500W) , control the temperature at 10-20°C and stir for 12 ho...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com