Compositions for treatment of fibrosis
A technology of fibrosis and compounds, applied in drug combination, active ingredient of anhydride/acid/halide, drug delivery, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
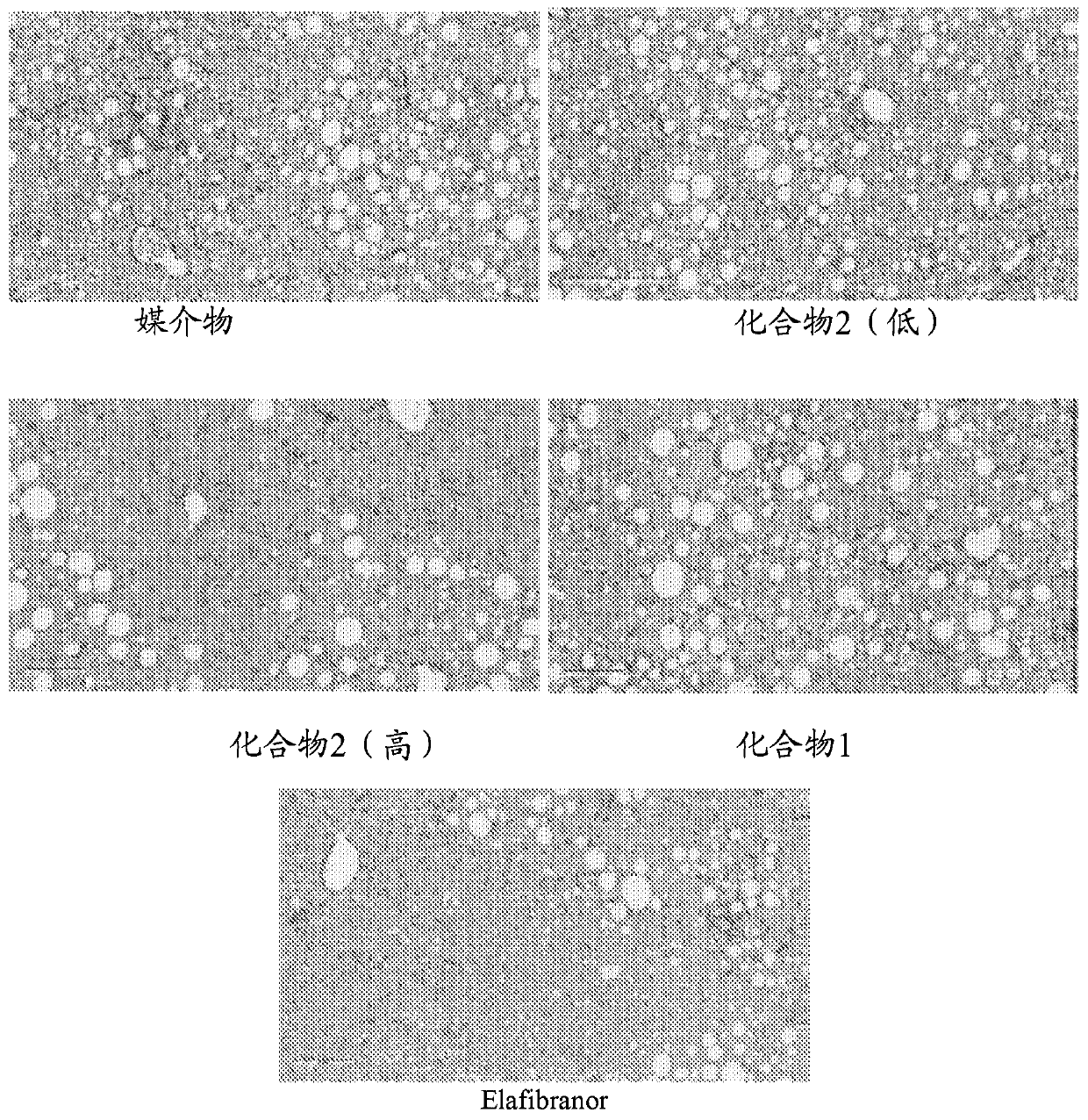
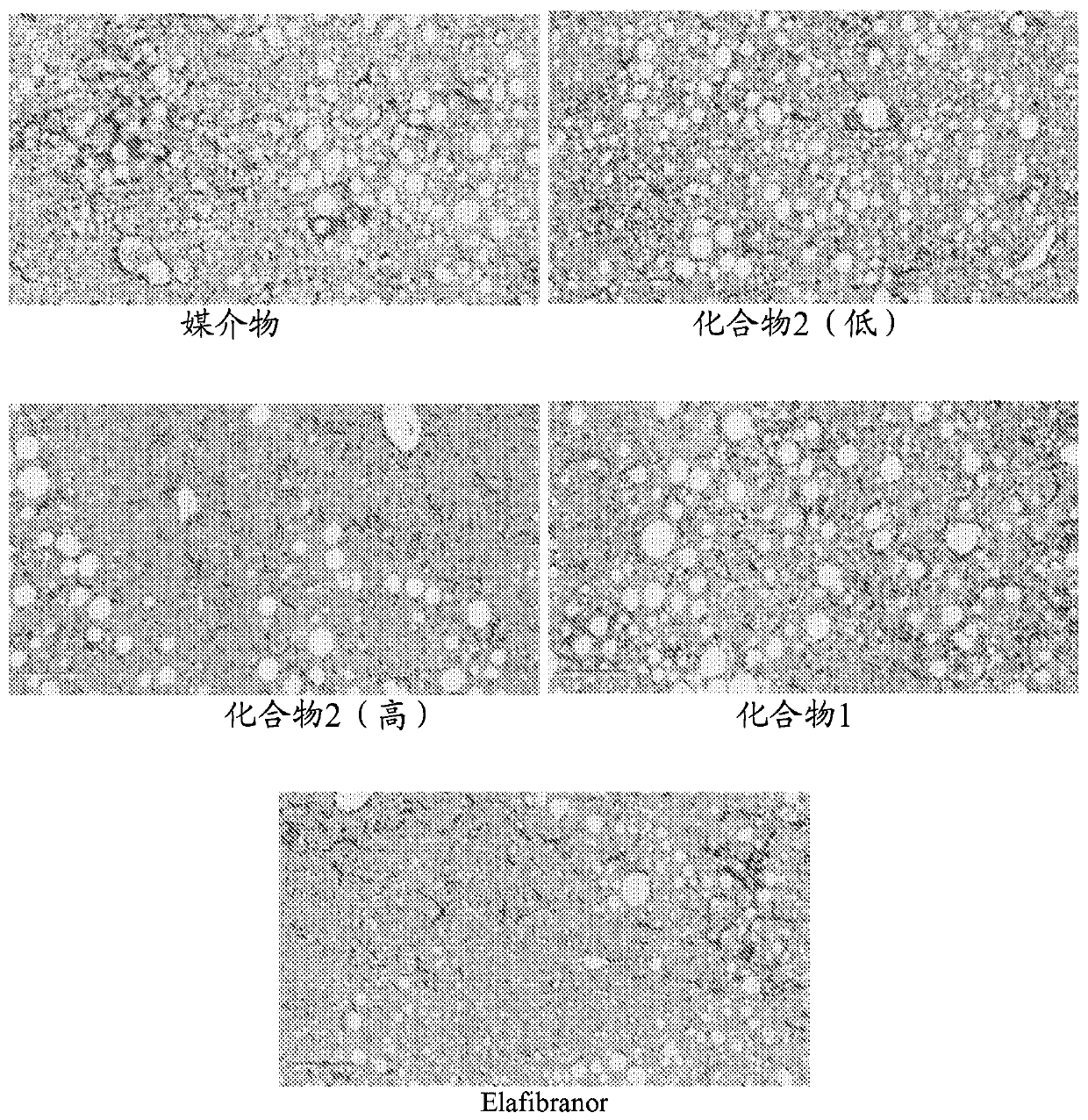
[0207] DIO-NASH mice were acclimatized for 3 weeks, and preconditioned liver biopsy samples were collected prior to acclimation. Mice were randomly assigned to one of 5 dose groups of 12 mice each. The doses assigned were: Compound 2 (low): 3 mg / kg; Compound 2 (high): 10 mg / kg; Compound 1: 10 mg / kg; and Elafibranor (30 mg.kg). One group was mock-treated with vehicle only as a control. The dosage form is administered orally once daily. After 8 weeks, animals were sacrificed and liver samples were taken. Liver samples were assayed for total hepatic hydroxyproline and subjected to immunohistochemical observation for the stage of fibrosis and the extent of Colal (collagen type Ia) staining. Such as figure 1 As shown in , Compound 2-treated animals showed lower total hepatic hydroxyproline levels than control-treated or mock-treated animals. Since hydroxyproline is an important component of collagen, and collagen is the most important source of hydroxyproline in animal tissues...
Embodiment 2
[0211] Pulmonary fibrosis was induced in healthy male Dunkin-Hartley guinea pigs by intratracheal administration of bleomycin. Control individuals were developed by intratracheal administration of saline solution. After establishment of pulmonary fibrosis in bleomycin-treated animals, a test article comprising any of Compounds 1 to 4 or any other compound disclosed herein was administered daily or as appropriate to each of the bleomycin-treated animals. individual for 6-10 weeks. Unilateral lung biopsies were taken prior to the first test article administration and again after sacrifice following the last test article administration. Biopsy samples were analyzed as described in Example I, plus immunohistochemical staining for type III collagen. Lungs from animals treated with the compounds disclosed herein, particularly those animals treated with Compound 2, exhibited decreased hydroxyproline levels, decreased collagen III staining, and decreased fibrosis relative to levels ...
Embodiment 3
[0215] Hypertrophy was induced in Sprague-Dawley rats by subcutaneous injection of capsaicin as described in Wallengren, J. et al., Skin Pharm. Appl. Skin Physiol. 15(3):154-165 (2002) Skin lesions, which are incorporated herein by reference for their description of inducing hypertrophic skin lesions in rats; or as described in Alonso-Merino et al., Proc. ), subcutaneous injection of CCl 4 and / or bleomycin induce hypertrophic skin lesions in C57BL or other appropriately stained mice, the disclosures of which are incorporated herein by reference for the induction of fibrotic skin lesions in mice. In capsaicin, CCl 4 and / or after establishment of hypertrophic skin lesions in bleomycin-treated animals, test articles containing any of Compounds 1 to 4 or any other compound disclosed herein are administered daily or as appropriate in a manner appropriate to their formulation. Administration to each individual animal continued for 6-10 weeks. Skin biopsies from the injection site...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com