Preparation method of 3-carboxybenzaldehyde
A technology of carboxybenzaldehyde and toluic acid, which is applied in the preparation of carboxylate, organic compound, and nitrile, can solve the problems of lack of preparation methods and 3-carboxybenzaldehyde, and achieve process operation Simple, low cost, high product purity and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
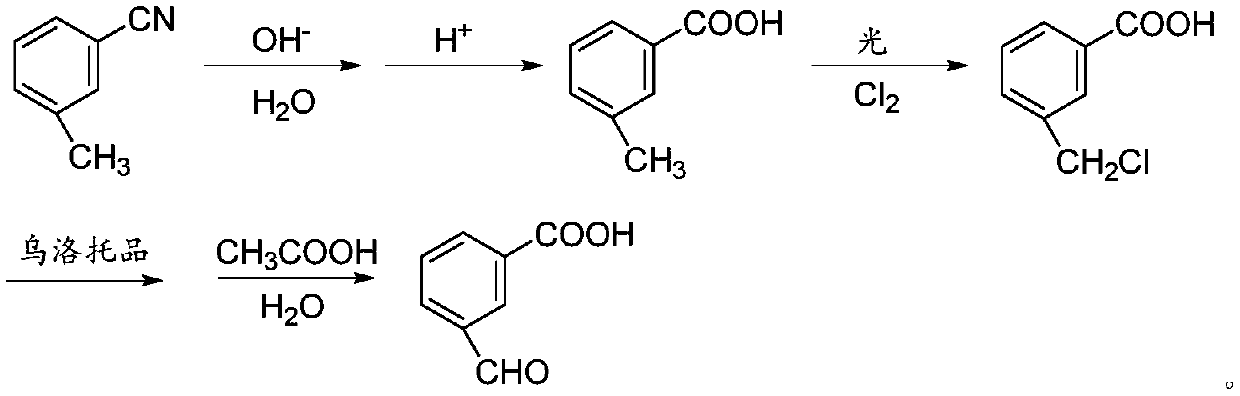
[0014] The invention provides a preparation method of 3-carboxybenzaldehyde, using m-tolunitrile as a starting material to obtain 3-carboxybenzene through the first hydrolysis reaction, acidification reaction, chlorination reaction, oxidation reaction and second hydrolysis reaction formaldehyde.
[0015] The preparation method of 3-carboxybenzaldehyde provided by the present invention adopts m-tolunitrile as the starting raw material and only needs to carry out simple first hydrolysis reaction, acidification reaction, chlorination reaction, oxidation reaction and second hydrolysis reaction to obtain , the reaction conditions are mild, the product purity and yield are high, the raw materials are simple and easy to obtain, and the production cost is greatly reduced.
[0016] In the second hydrolysis reaction process of the present invention, glacial acetic acid is selected as a raw material. Compared with other acids, glacial acetic acid is selected to make the hydrolysis reacti...
Embodiment 1
[0041] Add 117g m-tolunitrile, 800g water, and 200g sodium hydroxide solution with a mass fraction of 30% to a 2000ml four-neck flask equipped with mechanical stirring, thermometer, reflux condenser, and heating device, start stirring, and heat up to 100°C for reflux React for 4 hours; then add dropwise 30% hydrochloric acid to adjust the pH to 1, cool to room temperature, filter, wash with water, filter and dry to obtain 134 g of m-toluic acid, the reaction yield of this step is 98.5%.
[0042] Put 68g of m-toluic acid and 680g of carbon tetrachloride into a 1000ml four-necked bottle with mechanical stirring, thermometer, reflux condenser, tail gas absorption device, chlorine pipe, and heating device, start stirring, and heat up to 120°C for reflux. Under the irradiation of a mercury lamp, feed chlorine gas evenly, the flow rate is 0.6l / min, react until the m-toluic acid disappears in the sampling, cool and filter, dry to obtain 81g of 3-carboxybenzyl chloride, the yield is 9...
Embodiment 2
[0046] Add 117g m-tolunitrile, 850g water, and 200g sodium hydroxide solution with a mass fraction of 35% to a 2000ml four-necked flask equipped with mechanical stirring, thermometer, reflux condenser, and heating device, start stirring, and heat up to 105°C for reflux React for 6 hours; then add 30% hydrochloric acid dropwise to adjust the pH to 1, cool to room temperature, filter, wash with water, filter and dry to obtain 131 g of m-toluic acid, the reaction yield of this step is 96.3%.
[0047] Put 68g of m-toluic acid and 680g of carbon tetrachloride into a 1000ml four-necked bottle with mechanical stirring, thermometer, reflux condenser, tail gas absorption device, chlorine pipe, and heating device, start stirring, and heat up to 125°C for reflux. Under the irradiation of a mercury lamp, feed chlorine gas evenly, the flow rate is 0.55l / min, react until the m-toluic acid in the sampling disappears, cool and filter, dry to obtain 76g of 3-carboxybenzyl chloride, the yield is...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com