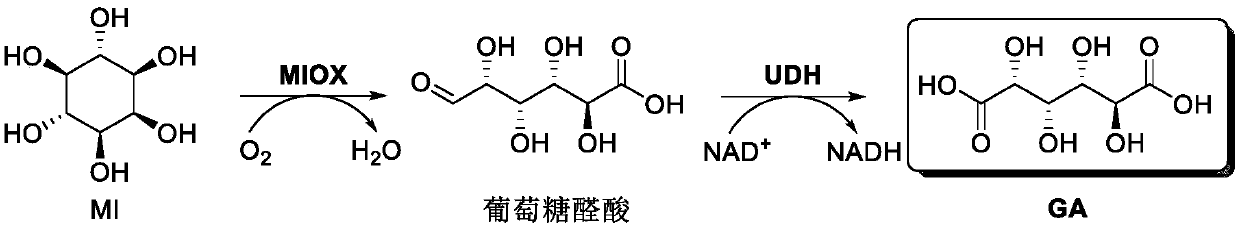
Preparation method of glucaric acid
A technology of glucaric acid and glucuronic acid is applied in the preparation of glucaric acid, the field of "one-pot-two-step" enzyme-catalyzed preparation of glucaric acid, which can solve the problem of low utilization rate of raw materials, degradation of xylan, and increasing process Complexity and other problems, to achieve the effect of high utilization rate of raw materials, prevention of product inhibition, and green-friendly yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Embodiment 1 prepares glucaric acid by inositol (1.8g / L)
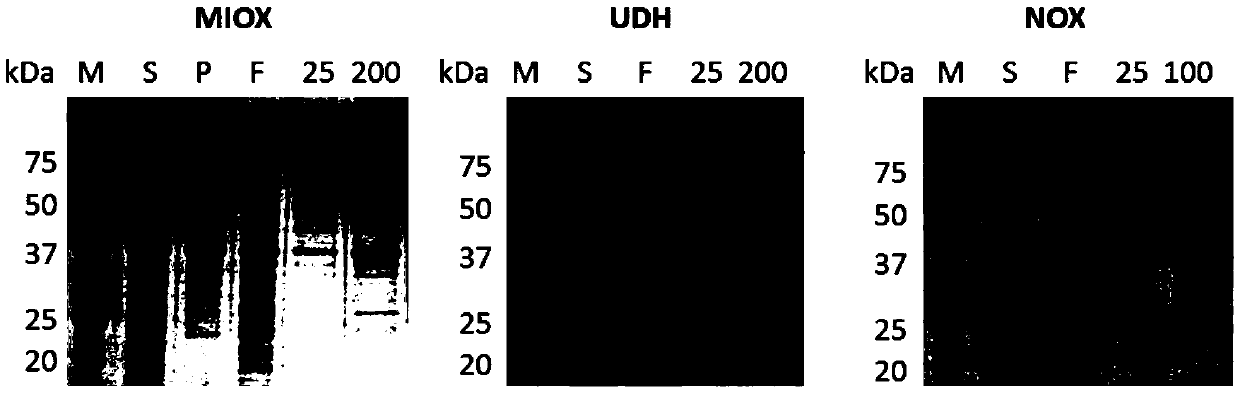
[0048] In this example, the inositol oxidase is from Cryptococcus neoformans, and the NCBI accession number is AAN85573.1. The gene miox was synthesized by BGI and optimized for codons. In this example, the aldolate dehydrogenase is from Agrobacterium tumefaciens, and the NCBI accession number is DAA06454.1. Agrobacterium tumefaciens was purchased from China General Microorganism Culture Collection Center (CGMCC). The gene udh was obtained from genomic DNA by PCR using corresponding primers. The above genes were respectively cloned into pET21a vectors by enzyme-linking or simple cloning methods to obtain the corresponding recombinant expression vectors pET21a-MIOX and pET21a-UDH. The above plasmids were respectively transferred into Escherichiacoli BL21 (DE3), and the protein expression and purification were carried out. The protein expression results were as follows: figure 2 shown.
[0049] (1) Inositol ...
Embodiment 2
[0056] Embodiment 2 utilizes NAD + Regeneration of glucaric acid from inositol (1.8g / L)
[0057] Utilize NAD + Regeneration of the catalytic pathway for the production of glucaric acid from inositol as Figure 4 shown.
[0058] The preparation method of inositol oxidase and aldolase dehydrogenase is the same as that in Example 1. In this example, the NADH oxidase is from Streptococcus mutans, and the NCBI accession number is BAA08707.1. The gene nox was synthesized by BGI and optimized for codons. The gene was cloned into the pET29a vector by restriction linking or Simple cloning to obtain the corresponding recombinant expression vector pET29a-NOX. The above plasmids were respectively transformed into E.coli BL21(DE3), and the protein expression and purification were carried out. The protein expression results were as follows: figure 2 shown.
[0059] (1) Inositol oxidase activation
[0060]A mixture containing 50 mM MOPS buffer (pH 6.5), 5 g / L inositol oxidase, 2.0 m...
Embodiment 3
[0066] Embodiment 3 utilizes NAD + Regeneration of glucaric acid from inositol (9g / L)
[0067] The preparation method of inositol oxidase and aldolase dehydrogenase is the same as that in Example 1. The preparation method of NADH oxidase is the same as that in Example 2. The initial concentration of inositol was 9g / L.
[0068] (1) Inositol oxidase activation
[0069] A mixture containing 50 mM MOPS buffer (pH 6.5), 10 g / L inositol oxidase, 2.0 mM ammonium ferrous sulfate and 5.0 mM ascorbic acid was incubated in an ice-water bath for 60 min to activate inositol oxidase.
[0070] (2) Generation of intermediate glucuronic acid
[0071] A 0.2 mL reaction system containing 200 mM MOPS buffer (pH 7.5), 9 g / L inositol and 2.1 g / L activated inositol oxidase was reacted at 30° C. and 1000 rpm. Use high performance liquid chromatography (HPLC) to detect until the output of intermediate glucuronic acid no longer changes. HPLC detection condition is the same as embodiment 1.
[00...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com