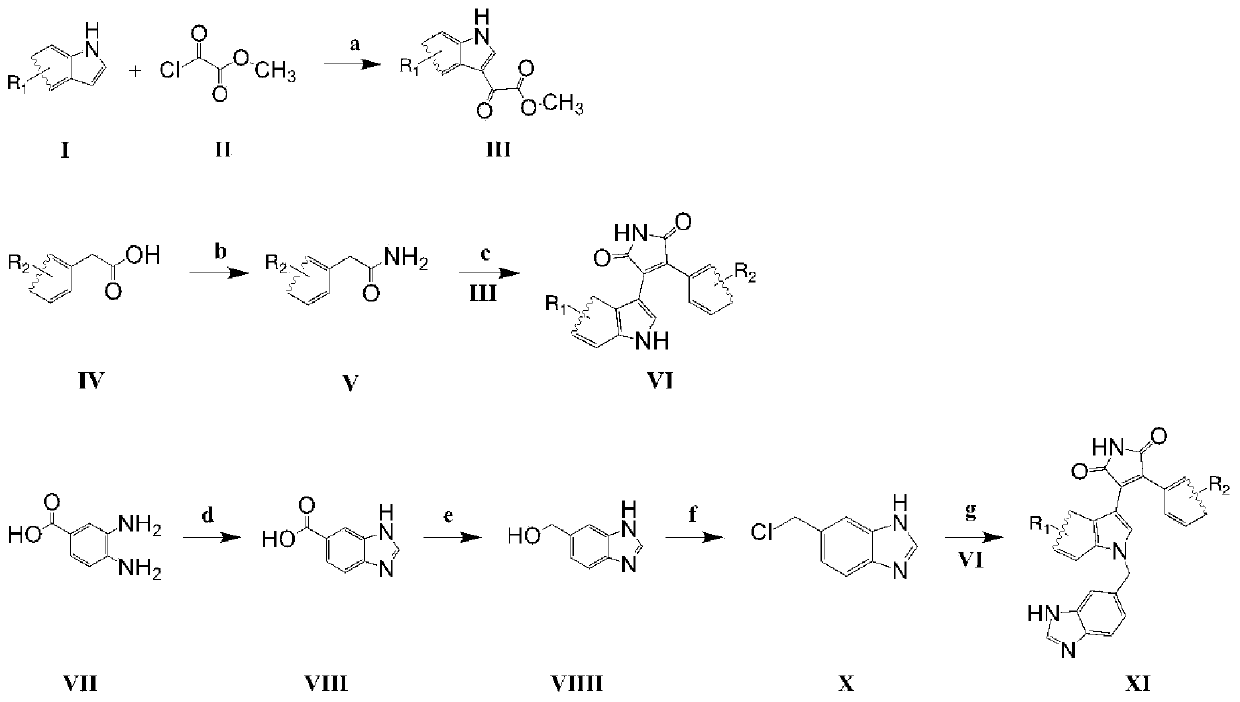
Preparation method and application of multi-target inhibitor acting on QC and GSK-3beta
An inhibitor, multi-targeting technology, applied in the field of preparation of multi-targeting inhibitors, can solve the problems of limited molecular activity, low drugability of lead compounds, insufficient molecular structure diversity, etc., and achieve the effect of expanding structural diversity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] figure 1 It is a schematic diagram of the synthetic route of the multi-target inhibitor in this example.
[0048] (1) Synthesis of intermediate product III:
[0049] a, with anhydrous CH 2 Cl 2 As a solvent, add 1 mole of raw material I, and add no less than 8 moles of anhydrous AlCl 3 , after stirring for 0.5h, dropwise add no less than 3 moles of methoxy oxalyl chloride solution II, stir at room temperature for 6-10h, add excess saturated NaHCO 3 solution, extracted 3 times with ethyl acetate, combined the organic phases, and used anhydrous Na 2 SO 4 Drying, concentration, and column chromatography to prepare product III, calculate the yield, and identify the structure;
[0050] (2) Synthesis of intermediate product VI:
[0051] b. With anhydrous CH 2 Cl 2 As a solvent, cool in an ice bath, add 1 mole of raw material IV, stir to dissolve, dropwise add not less than 4 moles of oxalyl chloride solution, dropwise add 2 drops of DMF, stir to room temperature, rea...
Embodiment 2
[0059] 3-(5-((1H-Benzo[d]imidazole-5-yl)methyl)-5H-pyrrolo[3,2-d]pyrimidine-7-yl)-4-phenyl-1H-pyrrole Preparation of -2,5-dione:
[0060] a. 20ml anhydrous CH 2 Cl 2 Add 0.105Mol 5H-pyrrolo[3,2-d]pyrimidine, add 1.107Mol anhydrous AlCl 3 , after stirring for 0.5h, add dropwise 0.439Mol methoxy oxalyl chloride solution, stir at room temperature for 6.5h, add 30ml saturated NaHCO 3 solution, extracted with ethyl acetate 20ml × 3 times, combined organic phase, anhydrous Na 2 SO 4 Drying, concentration, and column chromatography prepared methyl 2-oxo-2(5H-pyrrolo[3,2-d]pyrimidin-7-yl)acetate in a yield of 72%;
[0061] b. 20ml of anhydrous CH cooled in an ice bath 2 Cl 2 Add 0.114Mol phenylacetic acid, stir to dissolve, dropwise add 0.524Mol oxalyl chloride solution, dropwise add 2 drops of DMF, stir to room temperature, react for 3h, evaporate the solvent, add 15ml of anhydrous THF, cool in an ice bath, dropwise add 60ml of concentrated ammonia water , reacted for 0.5h, a...
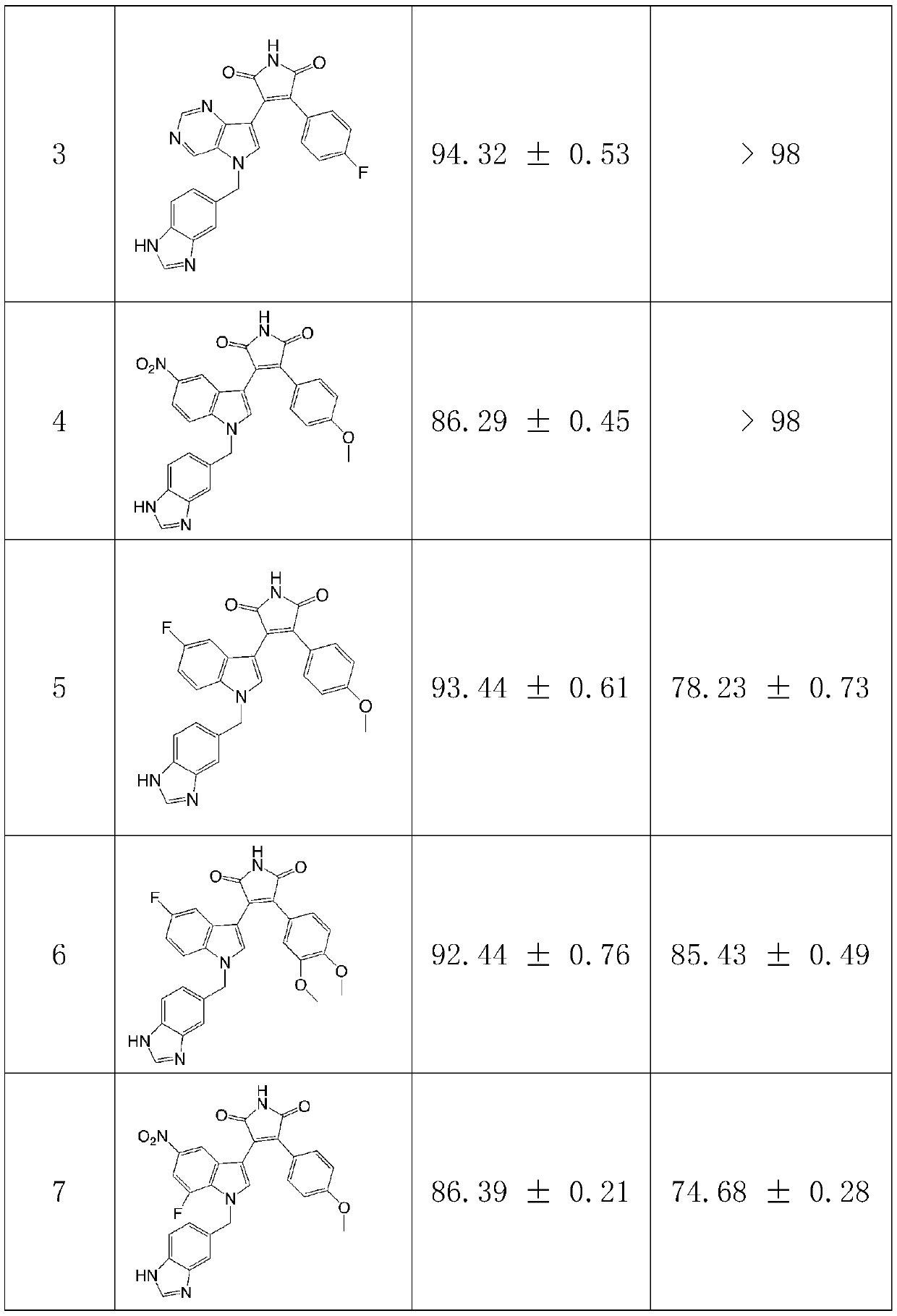
Embodiment 3
[0068] 3-(1-((1H-Benzo[d]imidazole-5-yl)methyl)-5-nitro-1H-indole-3-yl)-4-(4-methoxyphenyl) - Preparation of 1H-pyrrole-2,5-dione:
[0069] a. 20ml anhydrous CH 2 Cl 2 Add 0.112Mol 5-nitro-1H-indole, add 1.326Mol anhydrous AlCl 3 , after stirring for 0.5h, dropwise add 0.513Mol methoxy oxalyl chloride solution, stir at room temperature for 8h, add 30ml saturated NaHCO 3 solution, extracted with ethyl acetate 20ml × 3 times, combined organic phase, anhydrous Na 2 SO 4 Drying, concentration, and column chromatography prepared 2-(5-nitro-1H-indol-3-yl)-2-oxoacetic acid methyl ester with a yield of 53%;
[0070] b. 20ml of anhydrous CH cooled in an ice bath 2 Cl 2 Add 0.108Mol p-methoxyphenylacetic acid, stir to dissolve, add dropwise 0.493Mol oxalyl chloride solution, add dropwise 2 drops of DMF, stir to room temperature, react for 2.5h, evaporate the solvent, add 15ml of anhydrous THF, and cool in an ice bath. Add 60ml concentrated ammonia water dropwise, react for 1h, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com