Controllable release system
A conveying system and equipment technology, applied in the field of controllable release systems, can solve the problems of inability to respond in a timely manner to the transmission of thrust, uneven transmission of force, and easy occurrence of off-axis, so as to avoid knotting or intertwining, Avoid knots or tangles and achieve controllable effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
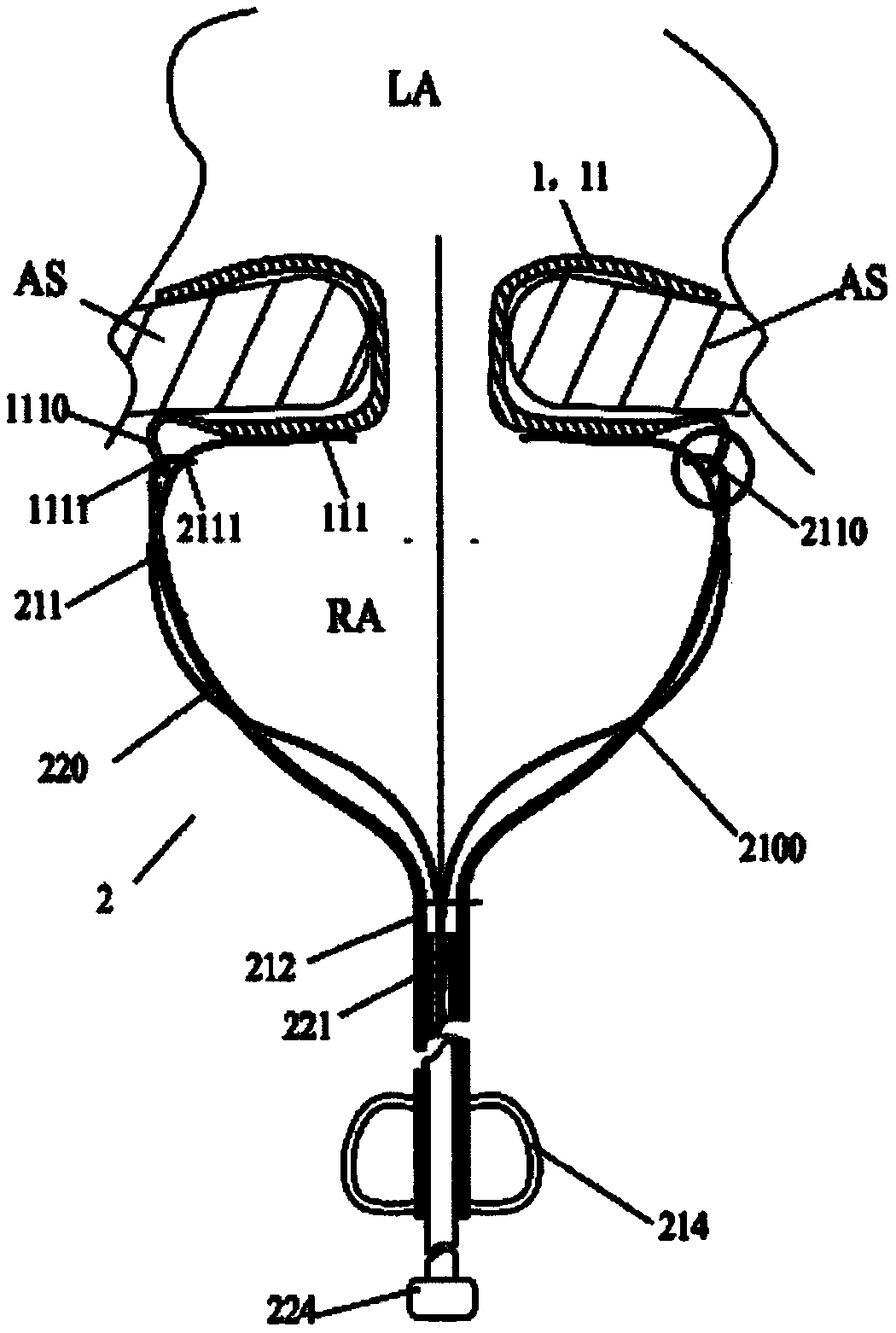
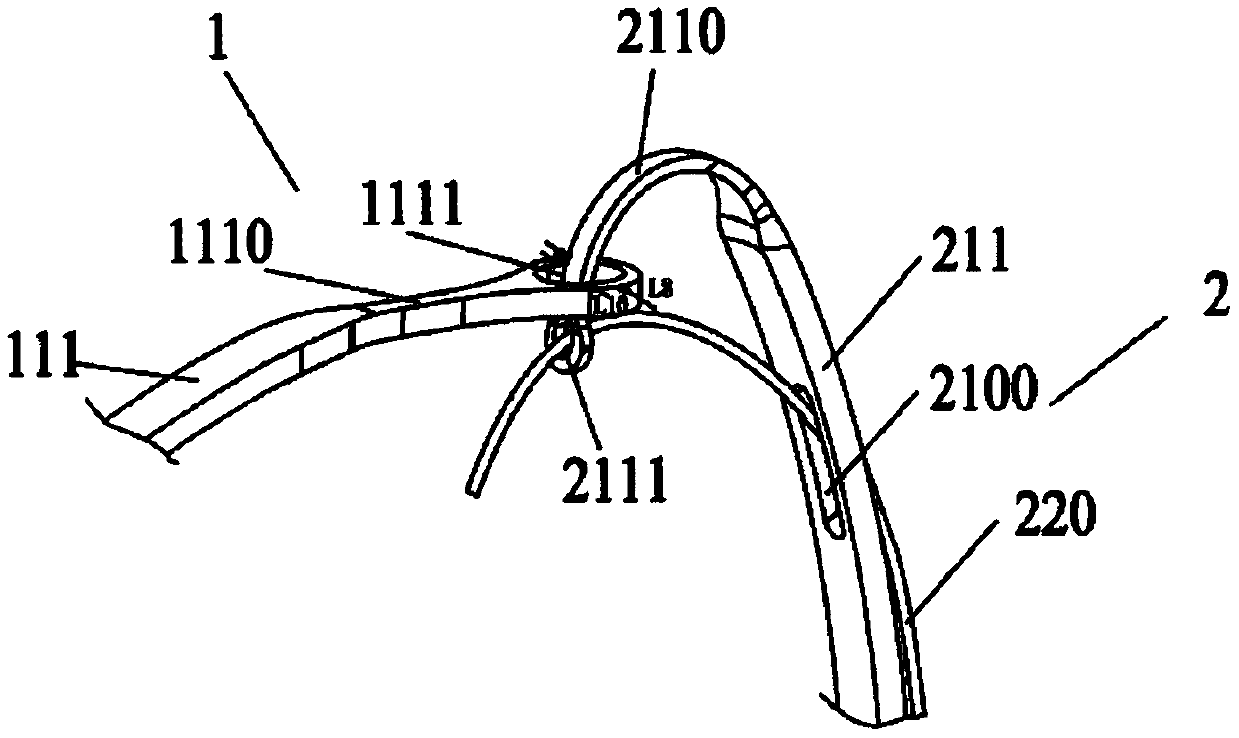
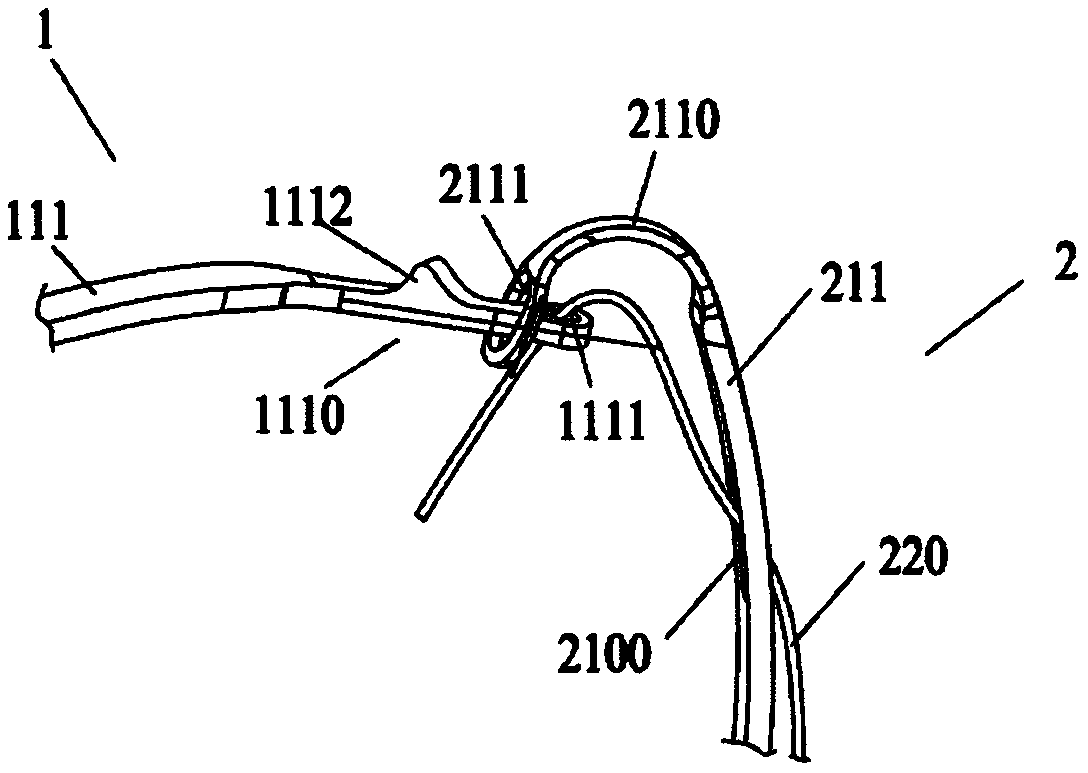
[0071] Such as figure 1 As shown, the controlled release system provided by the present invention includes an implant device 1 and a delivery system 2 . The proximal region of the implant instrument 1 is provided with a plurality of first holes 1111, and the delivery system 2 includes a plurality of traction rods 211 and a plurality of release wires 220, a plurality of release wires 220, a plurality of traction rods 211 and a plurality of first holes 1111 correspond to each other in quantity and position, and each traction rod 211 is provided with a second hole 2111 and a binding member 210, the second hole 2111 is located at the far end of the traction rod 211, and the binding member 210 is located in the second hole 2111 The proximal end side of the device, the first hole 1111 provided on the implant instrument 1 and the second hole 2111 provided on the traction rod 211 can interpenetrate with each other, and the release wire 220 passes through the binding member 210, the fi...
Embodiment 2
[0095] On the basis of Embodiment 1, as a special implementation example, the implant device 1 is an elastic ostomy stent 11 that can be placed at the interatrial septum, and the delivery system 2 is used to transfer the elastic ostomy stent 11 from Delivered in vitro with controlled release into the atrial septum. Specifically, the elastic pore-making scaffold 11 is a three-dimensional corrugated and / or net-like structure formed by interconnecting a plurality of support rods 111, and the three-dimensional corrugated and / or net-like structure includes a left disc that fits with the interatrial septal surface in the left atrium cavity. , the right disc that fits the interatrial septum in the right atrium cavity, and the waist that is arranged between the left disc and the right disc and is fixedly connected to the left disc and the right disc, and a through hole is arranged in the waist, and the through hole makes the left disc The atrium and the right atrium can be fluidly con...
Embodiment 3
[0110] Based on Embodiment 1, the difference between Embodiment 3 and Embodiment 1 is that the restraint member 210 is not a restraint hole 2100 provided on the drawbar 211 , but a restraint pipe 2101 or restraint ring 2102 is provided on the drawbar 211 .
[0111] In one embodiment, as Figures 4a-4c As shown, the binding member 210 is a binding tube 2101 with a hollow tubular structure, and the binding tube 2101 is sleeved outside the drawbar 211. The binding tube 2101 is preferably made of polymer tubes such as FEP, PTFE, PE, POE, PET, and silica gel. It can accommodate at least one traction rod 211 and one release wire 220 at the same time, which not only enhances the fit between the release wire 220 and the draw rod 211, but also enables the release wire 220 to pass through the lumen of the restraint tube 2101 smoothly and can be placed in the restraint tube 2101 At the same time, avoid opening holes on the traction rod 211, to a certain extent increase the anti-deformat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com