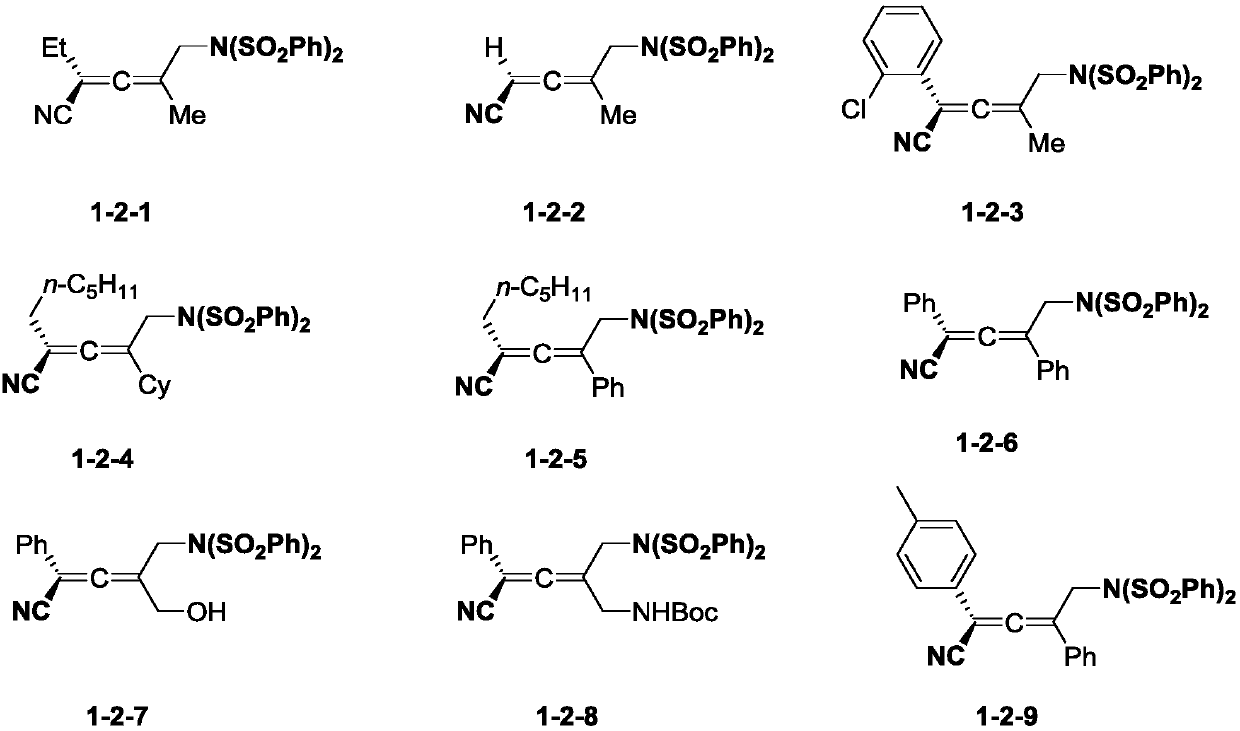
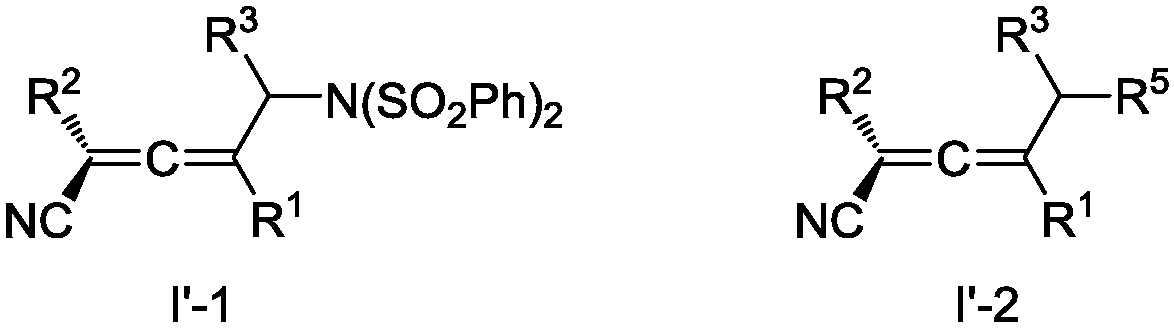Preparation method of allene compound
A compound and alkyl technology, applied in the field of preparation of allene compounds, can solve the problems of difficulty in utilization, short life, lack of convenient methods, etc., and achieve the effects of good stereoselectivity and wide substrate adaptability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055]
[0056]In this example, the compound represented by the general formula 1-2 was prepared according to the following preparation method and proportion: under the protection of nitrogen, add CuTc (0.01mmol), 1,10-phenanthroline (0.013mmol) into a 25mL reaction tube , evacuated, replaced with nitrogen three times, added acetonitrile (1 mL), and stirred at room temperature for 30 minutes. Add 1,3-enyne (0.2mmol), NFSI (0.3mmol) and TMSCN (0.3mmol) represented by formula 1-1 into the reaction tube, and react at room temperature for 12 hours. After the reaction is completed, dilute with ethyl acetate, The silica gel was quickly filtered, and the solvent was removed by a rotary evaporator under reduced pressure, followed by column chromatography to separate the obtained product sample as the compound of the general formula 1-2.
[0057] The compound shown in general formula 1-2 has following structure:
[0058]
Embodiment 2
[0060]
[0061] In this example, the compound represented by the general formula 2-2 was prepared according to the following preparation method and proportion: under the protection of nitrogen, add CuTc (0.01mmol), 1,10-phenanthroline (0.013) into a 25mL reaction tube , evacuated, replaced with nitrogen three times, added acetonitrile (1 mL), and stirred at room temperature for 30 minutes. Add 1,3-enyne (0.2mmol), NFSI (0.3mmol) and TMSCN (0.3mmol) shown in formula 2-1 into the reaction tube, and react at 50 degrees Celsius for 12 hours. After the reaction is completed, dilute with ethyl acetate , quickly filtered through silica gel, and the solvent was removed by a rotary evaporator under reduced pressure, and separated by column chromatography, and the product sample obtained was designated as compound 2-2. The compound shown in general formula 2-2 has following structure:
[0062]
[0063]
Embodiment 3
[0065]
[0066] In this example, the compound shown in the general formula 3-2 was prepared according to the following preparation method and proportion: under the protection of nitrogen, the Cu(OAc) 2 (0.025mmol, 5mol%), 1,10-phenanthroline (0.0325mmol, 6.5mol%) were added to a 25mL reaction tube, dichloromethane (2mL) was added, and stirred at room temperature for 30 minutes. Add 1,3-enyne (0.5mmol, 1.0eq), peroxide (1.5mmol, 3.0eq), TMSCN (1.5mmol, 3eq) shown in Formula 3-1 into the reaction tube, and react at 50°C After 12 hours, after the completion of the reaction, dilute with ethyl acetate, quickly filter through silica gel, remove the solvent under reduced pressure with a rotary evaporator, and separate by column chromatography to obtain a product sample as compound 3-2.
[0067] The compound shown in general formula 3-2 has following structure:
[0068]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com