Ganoderma lucidum sporocarp microbial fermentation preparation for treating intestinal flora disorder and intestinal barrier function impairment
A technology of Ganoderma lucidum fruiting body and microbial fermentation, applied in the field of fermentation engineering, to improve the effect of impaired intestinal barrier function
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] A preparation method of ganoderma lucidum fruiting body microbial fermentation preparation for treating intestinal flora disorder and impaired intestinal barrier function, the steps are as follows:
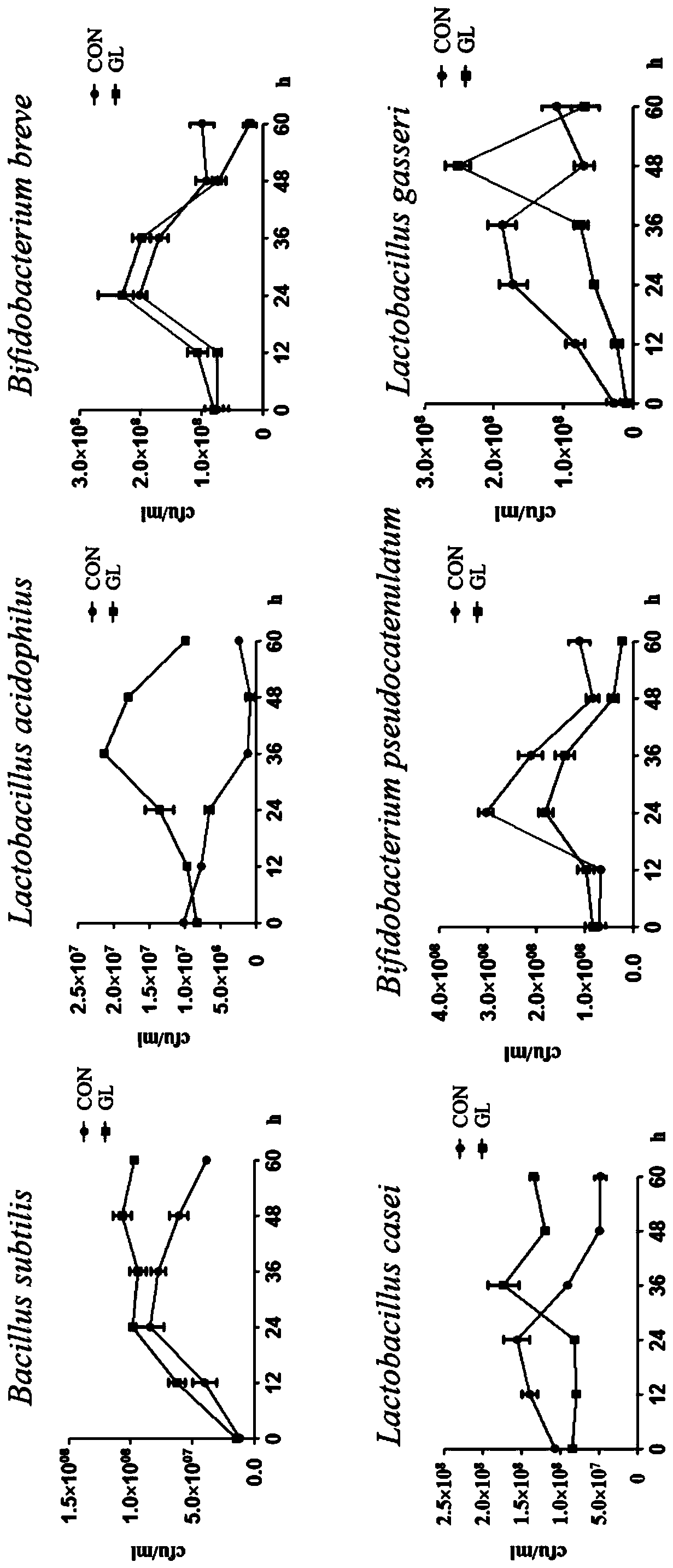
[0035] 1) Screening of strains: Screen the strains that can grow in Ganoderma lucidum medium from the following probiotic strains, including Bacillus subtilis, Lactobacillus acidophilus, Bifidobacterium breve, Lactobacillus casei, Pseudomonas pseudobacterium Bifidobacterium streptoides and Lactobacillus gasseri.
[0036] 2), preparation of MRS medium containing three concentrations of Ganoderma lucidum extract in low, medium and high concentrations
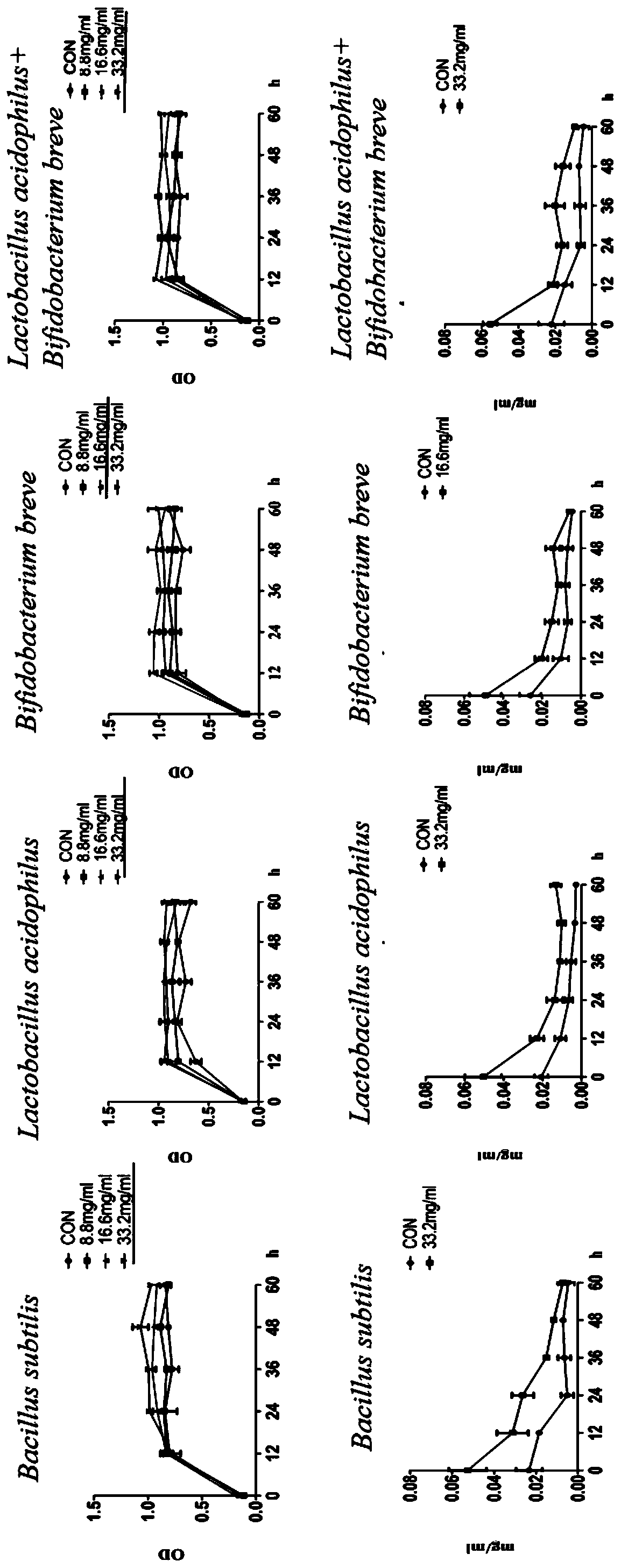
[0037] Ganoderma lucidum fruiting body powder was prepared with water into Ganoderma lucidum extracts with three concentrations of 8.8mg / ml, 16.6mg / ml, and 33.2mg / ml, and then added to MRS medium containing 0.5% sugar respectively, and processed under high pressure to produce MRS medium containing Ganoderma lucidum extract.
...
Embodiment 2
[0042] Determination of the growth of probiotics and the content of total sugar in probiotic fermentation broth of Ganoderma lucidum fruiting bodies.
[0043] Microbial growth: Measure the OD value at 620nm and draw the growth curve to obtain the growth of probiotics.
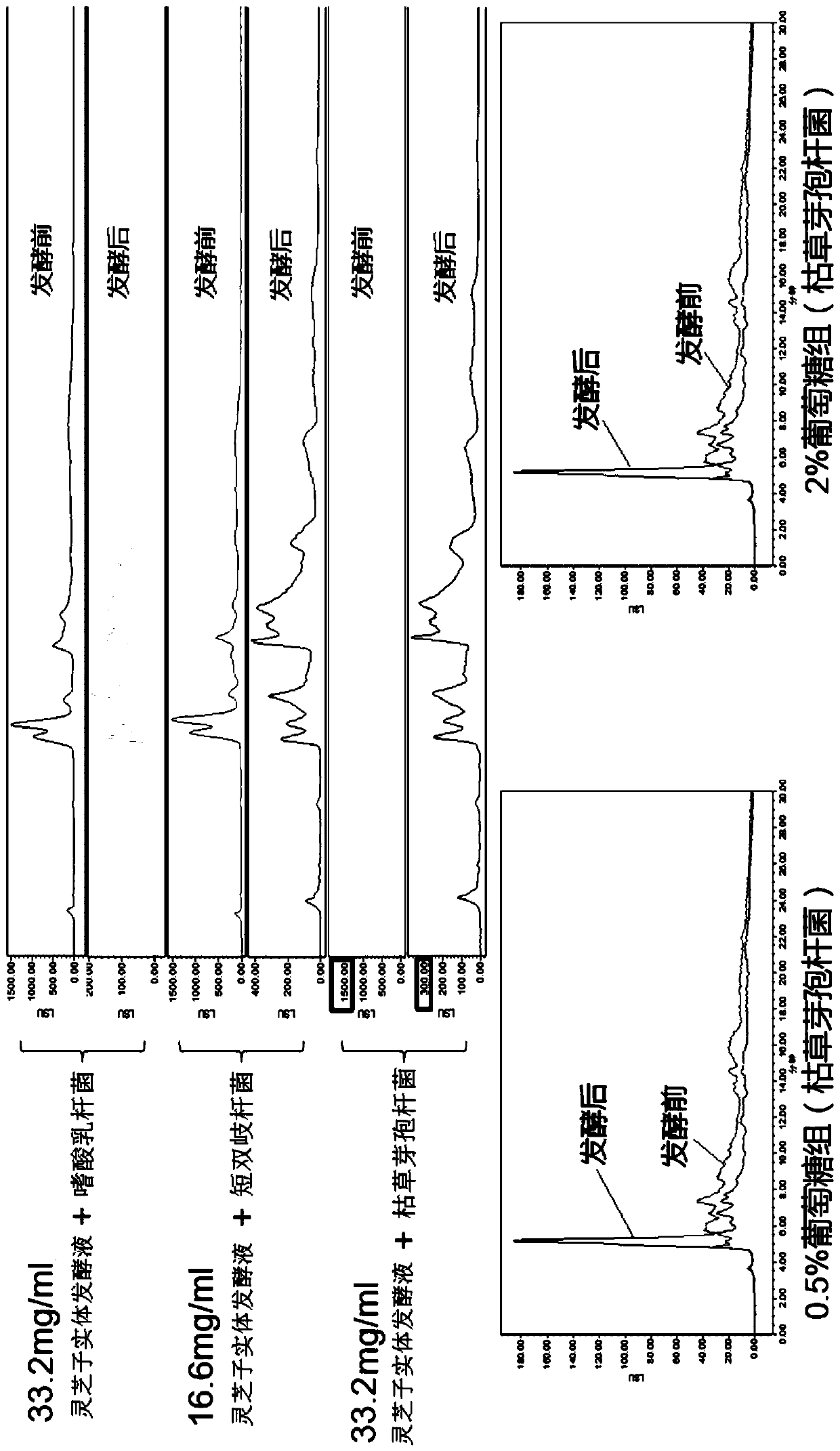
[0044] Determination of total sugar content: Glucose is the standard product, using the phenol-concentrated sulfuric acid method, take 6 clean test tubes and number them, draw 0, 0.2, 0.4, 0.6, 0.8, 1.0ml of 0.1mg / ml standard glucose solution into the test tubes respectively, Make up to 1ml with distilled water, add 1ml of 6% phenol and 5ml of concentrated sulfuric acid to each test tube, mix well and let stand for 10min. React in a water bath at 30°C for 20 minutes, take an appropriate amount of the reaction solution and measure the absorbance at 490 nm, draw a standard curve with glucose concentration (ug / ml) as the abscissa and absorbance as the ordinate. Take 2ml of fermentation broth, add 5ml of water, 1....
Embodiment 3
[0047] Application of Ganoderma lucidum fruiting body-Bacillus subtilis fermentation liquid (obtained by inoculation and fermentation of Bacillus subtilis (such as DM9610, etc.), the preparation method is the same as that in Example 1) in animal models of intestinal flora disturbance and impaired intestinal barrier function.
[0048] Mainly observe the changes in animal weight, feces, and cecal index; pathological detection of colon tissue HE staining; flow cytometry analysis of spleen CD3 + T cells, CD4 + T cells, CD8 + Changes of T cells and macrophages; ELISA double-antibody sandwich method to detect IL-10, IL-6, TNF-α, LPS; 16SrDNA high-throughput sequencing to detect intestinal flora. The specific steps are:
[0049]Male Balb / c mice aged 6-8 weeks were used to construct animal models and divided into 5 groups (7 mice in each group), which were control (CON) group, antibiotic (CS) group, fermentation broth treatment (FT) group, Fermentation fluid prevention (FP) group, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com