A kind of β2 receptor agonist inhalation aerosol and products containing the inhalation aerosol
A β2 receptor, aerosol technology, applied in the field of inhaled pharmaceutical preparations, can solve the problems of non-compliance with large-scale production of products, product dose uniformity and low product stability, achieve good market prospects, and improve effective lung deposition. , the effect of improving stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~5
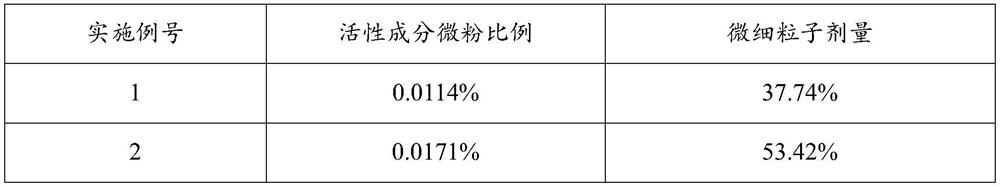
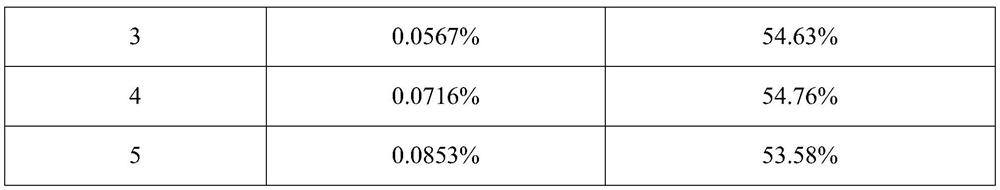
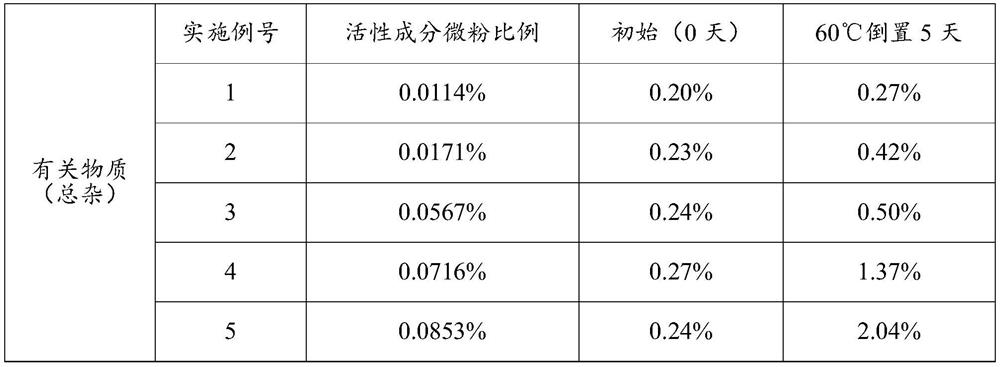
[0043] The addition amount of active ingredient micropowder is 0.0114%~0.0853% (w / w), the addition amount of anhydrous lactose micropowder is 0.0857% (w / w), the addition amount of PEG1000 is 0.0201% (w / w), and the addition amount of absolute ethanol is 0.9988% (w / w), the addition of PVPK25 is 0.000092% (w / w), the addition of propellant is 98.8099% to 98.8839% (w / w), and the combination of fluorocarbon coated metal tank and quantitative metal valve is used as the container System, each component is shown in Table 1 below:
[0044] Table 1. Prescription (w / w) of inhaled aerosols of Examples 1-5
[0045] Example number 1 2 3 4 5 Active Ingredient Micronized 0.0114% 0.0171% 0.0567% 0.0716% 0.0853% Anhydrous Lactose Powder 0.0857% 0.0857% 0.0857% 0.0857% 0.0857% PEG1000 0.0201% 0.0201% 0.0201% 0.0201% 0.0201% Absolute ethanol 0.9988% 0.9988% 0.9988% 0.9988% 0.9988% PVP K25 0.000092% 0.000092% 0.000092% 0.000092% 0.0000...
Embodiment 6~10
[0053] The addition amount of active ingredient micropowder is 0.0338% (w / w), the addition amount of anhydrous lactose micropowder is 0.0850%~0.3596% (w / w), the addition amount of PEG1000 is 0.0144% (w / w), and the addition amount of absolute ethanol is 0.5094% (w / w), the amount of PVPK25 added is 0.000131% (w / w), the amount of propellant added is 99.0827% ~ 99.3893% (w / w), and the combination of fluorocarbon coated metal tank and quantitative metal valve is used as the container System, each component is shown in Table 4 below:
[0054] Table 4. Prescription (w / w) of inhaled aerosols of Examples 6-10
[0055] Example number 6 7 8 9 10 Active Ingredient Micronized 0.0338% 0.0338% 0.0338% 0.0338% 0.0338% Anhydrous Lactose Powder 0.0850% 0.0857% 0.1271% 0.2573% 0.3596% PEG1000 0.0144% 0.0144% 0.0144% 0.0144% 0.0144% Absolute ethanol 0.5094% 0.5094% 0.5094% 0.5094% 0.5094% PVP K25 0.000131% 0.000131% 0.000131% 0.000131...
Embodiment 11~15
[0062] The addition amount of active ingredient micropowder is 0.0405% (w / w), the addition amount of anhydrous lactose micropowder is 0.1524% (w / w), the addition amount of PEG1000 is 0.0023%~0.0516% (w / w), and the addition amount of absolute ethanol is 1.2851% (w / w), the addition of PVP K25 is 0.000670% (w / w), the addition of propellant is 98.4697% to 98.5190% (w / w), and the combination of fluorocarbon coated metal tank and quantitative metal valve is used as For the container system, see the following table 7 for details of each component:
[0063] Table 7. Prescriptions (w / w) of inhalation aerosols of Examples 11-15
[0064] Example number 11 12 13 14 15 Active Ingredient Micronized 0.0405% 0.0405% 0.0405% 0.0405% 0.0405% Anhydrous Lactose Powder 0.1524% 0.1524% 0.1524% 0.1524% 0.1524% PEG1000 0.0023% 0.0051% 0.0208% 0.0409% 0.0516% Absolute ethanol 1.2851% 1.2851% 1.2851% 1.2851% 1.2851% PVP K25 0.000670% 0.000670...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com