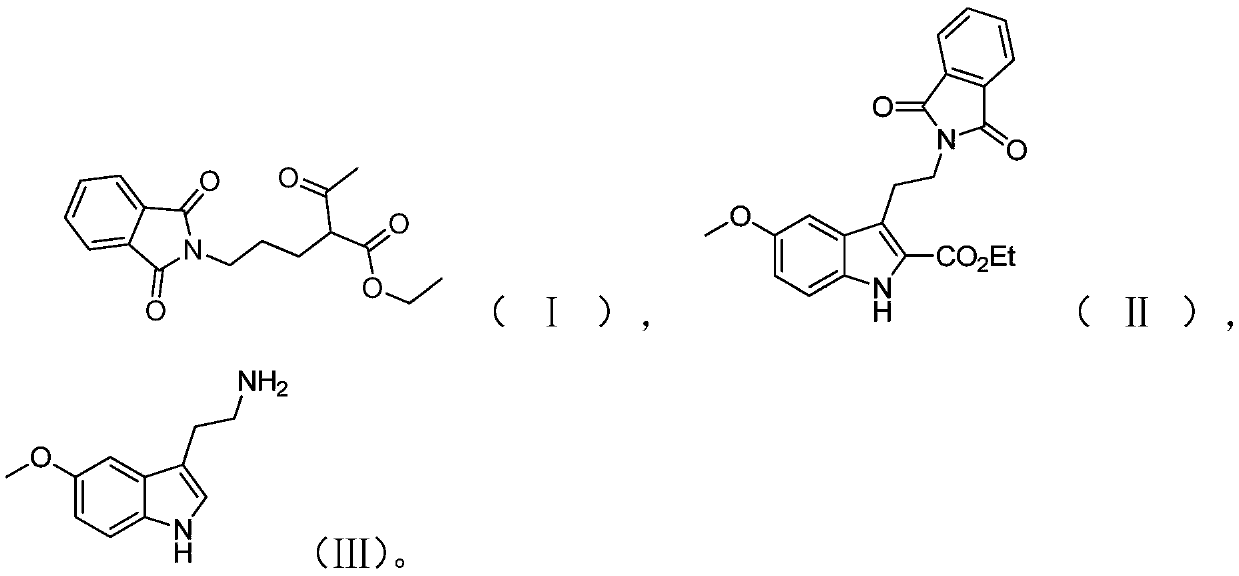
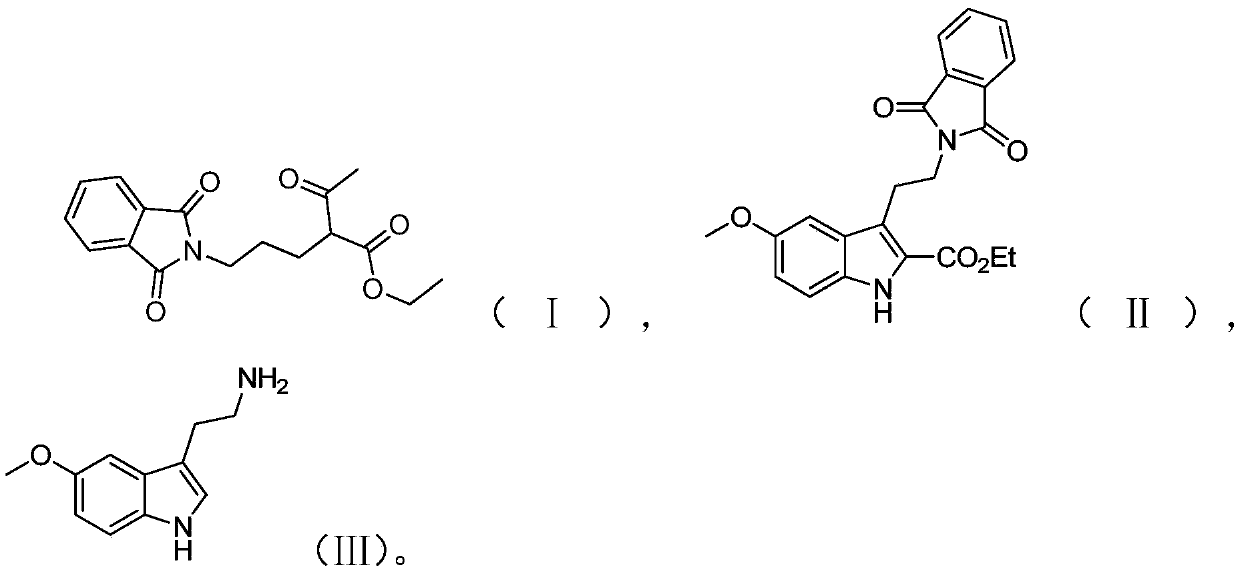
Preparation method of melatonin
A technology of melatonin and compounds, applied in the field of preparation of melatonin, can solve the problems of cumbersome steps, high risk and cost, reagent toxicity, etc., and achieve simple operation, shortened reaction steps and time, and high overall yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049]
[0050] The preparation route of melatonin provided in this embodiment is as shown above, and the preparation method comprises the following steps:
[0051] (1) Add 500 mL of acetonitrile, 100 g of phthalimide and 281.8 g of potassium carbonate to the reaction vessel, stir at room temperature for 30 min, then add 115.19 g of 1,3-dichloropropane and 152.82 g of sodium iodide; After the feeding is completed, gradually raise the temperature to 58-60°C and stir the reaction for 5.5 hours in this temperature range, and take samples to monitor the complete reaction of the raw material phthalimide;
[0052] Then 132.68 g of ethyl acetoacetate was weighed, and slowly added dropwise to the above reaction solution. After the addition, the reaction was continued for 7 h; was 96.2%.
[0053] (2) Add 100mL of water and 121g of p-methoxyaniline to the reaction vessel, cool down to 0-5°C; slowly add 298.53g of concentrated hydrochloric acid (36wt%) to it, and stir for 30min after...
Embodiment 2
[0060] The present embodiment refers to the preparation method of Example 1, the difference is only that step (1) is as follows:
[0061] Add 500mL acetonitrile, 100g phthalimide and 216.1g sodium carbonate to the reaction vessel, stir at room temperature for 30min, then add 115.19g of 1,3-dichloropropane and 152.82g sodium iodide; Gradually raise the temperature to 58-60°C and stir in this temperature range for 5.5 hours, take a sample to monitor the complete reaction of the raw material phthalimide;
[0062] Then 132.68 g of ethyl acetoacetate was weighed, and slowly added dropwise to the above reaction solution. After the addition, the reaction was continued for 7 h; was 90.7%.
Embodiment 3
[0064] The present embodiment refers to the preparation method of Example 1, the difference is only that step (1) is as follows:
[0065] Add 1000mL acetonitrile, 100g phthalimide and 375.7g potassium carbonate to the reaction vessel, stir at room temperature for 30min, then add 153.6g of 1,3-dichloropropane and 203.8g sodium iodide; Gradually raise the temperature to 58-60°C and stir in this temperature range for 5.5 hours, take a sample to monitor the complete reaction of the raw material phthalimide;
[0066] Then 176.9 g of ethyl acetoacetate was weighed and slowly added dropwise to the above reaction solution. After the addition was complete, the reaction was continued for 7 h; was 94.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com